Article Text
Abstract
Secondhand smoke exposure (SHSe) is a known cause of many adverse health effects in adults and children. Increasingly, SHSe assessment is an element of tobacco control research and implementation worldwide. In spite of decades of development of approaches to assess SHSe, there are still unresolved methodological issues; therefore, a multidisciplinary expert meeting was held to catalogue the approaches to assess SHSe and with the goal of providing a set of uniform methods for future use by investigators and thereby facilitate comparisons of findings across studies. The meeting, held at Johns Hopkins, in Baltimore, Maryland, USA, was supported by the Flight Attendant Medical Research Institute (FAMRI). A series of articles were developed to summarise what is known about self-reported, environmental and biological SHSe measurements. Non-smokers inhale toxicants in SHS, which are mainly products of combustion of organic materials and are not specific to tobacco smoke exposure. Biomarkers specific to SHSe are nicotine and its metabolites (eg, cotinine), and metabolites of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Cotinine is the preferred blood, saliva and urine biomarker for SHSe. Cotinine and nicotine can also be measured in hair and toenails. NNAL (4-[methylnitrosamino]-1-[3-pyridyl]-1-butanol), a metabolite of NNK, can be determined in the urine of SHS-exposed non-smokers. The selection of a particular biomarker of SHSe and the analytic biological medium depends on the scientific or public health question of interest, study design and setting, subjects, and funding. This manuscript summarises the scientific evidence on the use of biomarkers to measure SHSe, analytical methods, biological matrices and their interpretation.
- Secondhand smoke exposure
- questionnaires
- biological markers
- environmental exposure
- validation
- surveillance and monitoring
- prevalence
- human rights
- secondhand smoke
- smoking caused disease
- environmental tobacco smoke
- harm reduction
- product analysis
- carcinogens
- global health
- litigation
- carcinogens
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license. See: http://creativecommons.org/licenses/by-nc/3.0/ and http://creativecommons.org/licenses/by-nc/3.0/legalcode
Statistics from Altmetric.com
- Secondhand smoke exposure
- questionnaires
- biological markers
- environmental exposure
- validation
- surveillance and monitoring
- prevalence
- human rights
- secondhand smoke
- smoking caused disease
- environmental tobacco smoke
- harm reduction
- product analysis
- carcinogens
- global health
- litigation
- carcinogens
Introduction
This article presents the scientific evidence on the use of biomarkers of secondhand smoke exposure (SHSe), analytical methods, biological matrices and their interpretation. A biomarker for SHSe should be: (a) unique to SHSe; (b) easily detectable using analytic methods reproducible across laboratories; (c) reflect known toxic exposures or high correlation with such exposures; and (d) exhibit changed levels with a corresponding change in disease risk.1 Most toxicants in tobacco smoke result from combustion of organic materials and are not specific to SHSe.1 Metabolites of nicotine (cotinine, trans-3′-hydroxycotinine and their glucuronides, and nicotine glucuronide) and NNK (NNAL (4-[methylnitrosamino]-1-[3-pyridyl]-1-butanol) and its glucuronides) can be measured in SHS-exposed individuals, with high sensitivity in various biological matrices (table 1).2 ,3
Biomarkers of secondhand smoke exposure (SHSe), characteristics and cut-off points for distinguishing smokers from non-smokers
Selection of biomarkers of SHSe
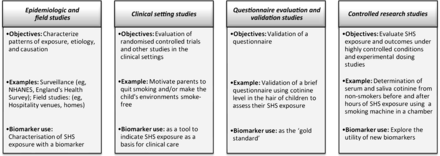
Selection of a SHSe biomarker depends on the scientific or public health question of interest, study design and setting, subjects, funding and laboratory access (figure 1). Novel biomarkers under development for use in highly controlled settings, such as chamber studies of exposures to volunteers, are not discussed here.
Types of study designs and biomarker use. NHANES, National Health and Nutrition Examination Survey; SHS, secondhand smoke.
Nicotine and metabolites
Nicotine is present in substantial concentrations in virtually all tobacco products and in insignificant amounts in some foods.4 ,5 Nicotine is extensively metabolised, primarily in the liver, and its major proximate metabolite is cotinine: on average, 75% of nicotine is converted to cotinine, primarily by the liver enzyme cytochrome P450 2A6.6 Cotinine's half-life (t1/2), the time in which its concentration halves, is longer (average: 16 h) than nicotine's (2 h). Cotinine concentrations are more stable throughout the day, making it the preferred blood, saliva and urine biomarker for SHSe (table 1). Blood's cotinine concentrations and saliva are highly correlated. Urine cotinine concentrations average fourfold to sixfold higher than those in blood or saliva, making urine a more sensitive matrix to detect low-concentration exposure.7 Six metabolites (nicotine, cotinine and trans-3′-hydroxycotinine (3-HC) and their respective glucuronide conjugates) account for about 85% to 90% of a nicotine dose, and the sum of these metabolites in urine provides an approximate estimate of daily nicotine intake.
Considerable between-individual variability exists in the rate and pattern of nicotine metabolism, possibly affecting cotinine concentration resulting from a given nicotine exposure. Factors influencing nicotine metabolism can include genetic variation, race, gender, oral contraceptive use or other oestrogen-containing hormones, kidney failure and drugs, including anticonvulsants and rifampin.6 Cotinine concentrations in biofluids and nicotine in hair are generally higher in infants and children, compared to SHS-exposed adults; this is probably due to greater inhaled nicotine doses (closer proximity to smokers and higher minute ventilation per body mass) and slower cotinine metabolism.8
NNK and metabolites
NNK is a nitrosamine and potent carcinogen formed primarily during tobacco curing, when nicotine or pseudo-oxynicotine reacts with nitrite in tobacco.9 NNK is metabolised in the body to NNAL and NNAL-glucuronides, commonly measured together, total NNAL (tNNAL). tNNAL remains in the body longer than cotinine (t1/2=10 days to 3 weeks) (table 1). NNAL is a potent lung carcinogen, with activity similar to NNK, and tNNAL detection in the urine of SHS-exposed non-smokers forms a biochemical link between exposure and lung cancer. In rats, NNK and NNAL are known to induce tumours of the pancreas, and NNK causes nasal mucosa and liver tumours, although at higher doses than for lung tumours.9
Concentrations of urine and plasma cotinine are highly correlated with urine tNNAL,10 ,11 although no data link these biomarkers to other SHS chemicals. The dynamic nature of SHS is critical when interpreting biomarkers for particular exposure patterns, including brief high-intensity versus sustained low-concentration exposures. Volatile compounds including nicotine leave the smoke and adsorb to room surfaces (eg, walls, floors, furniture) quickly, while other volatile compounds may persist in the air.12
Analytical methods for biomarkers of SHSe
Choosing a laboratory for analysis should occur early in a study to assure that the collection protocol is suited to the assay. Analysis actually begins with sampling, and the collection protocol may have significant implications for subsequent assays. Analytical methods include radioimmunoassay,13 ,14 ELISA,14 ,15 gas chromatography (GC)-nitrogen-phosphorous detection (NPD),16 GC-thermal energy analysis (GC-TEA),17 GC-mass spectrometry (GC-MS),18 liquid chromatography (LC)-electrochemical detection (ECD),19 LC-tandem mass spectrometry (LC-MS/MS) and GC-MS/MS20 (table 2).
Analytical methods for measurement of biomarkers of secondhand smoke exposure (SHSe)
Urine nicotine and metabolites
Urine cotinine is a widely used biomarker of SHSe, with the sum of free cotinine and cotinine glucuronide (conjugated) resulting in higher concentrations in some studies.10 ,24–27 For comparison purposes, researchers must consider whether free cotinine or total (free plus conjugated) cotinine was measured. Free cotinine is preferable to use as it correlates better with plasma cotinine than total cotinine.7 Current state-of-the-art methods are GC-MS/MS and LC-MS/MS, with limits of detection (LOD) of 0.05 ng/ml,20 ,21 a sensitivity concentration needed in countries with low SHSe due to clean air regulations and lower air nicotine concentrations.
The advantages of determining SHSe with urine are that cotinine concentrations and other metabolites are higher than in other biological fluids; it represents relatively acute exposure; and collection is non-invasive. Disadvantages include the facilities for privacy during collection, difficulty collecting in population-based or children studies, variability in cotinine conversion factor and needing to adjust for creatinine clearance (data on renal diseases and prescription drugs that interfere with metabolism and renal secretion).
Blood nicotine and metabolites
Nicotine, cotinine and 3-HC can all be measured in blood, but cotinine is generally the preferred biomarker4 because of its longer half-life.28 With regular, sustained exposures, the longer cotinine t1/2 results in higher, more uniform steady-state concentrations than nicotine. However, the cotinine t1/2 is <1 day, indicating only recent SHSe. Cotinine t1/2 may be longer in young children than in older children and adults,29 and slightly shorter in women than in men.30 This reduction is especially notable in pregnant women due to increased clearance rates,31 and is critical when evaluating relative concentrations and applying cotinine values to identify pregnant smokers and non-smokers.
Cotinine is commonly measured in serum or plasma, with comparable results from either matrix; although uncommon, whole blood may also be used. Current analytic methods require no more than 1 ml of serum, which is readily obtainable from adults, although infants and young children may present challenges. GC-MS/MS and LC-MS/MS are preferred analytical methods for these matrices.
A potential disadvantage of serum assays versus urine is lower sensitivity, as urine cotinine concentrations are typically fourfold to sixfold higher than in serum. If extremely sensitive analytic methods are used, however, this is not a concern. As serum does not require adjustment for hydration differences among individuals, it provides a more uniform matrix measurement than urine. Blood collection is invasive, however, and salivary cotinine may be a necessary alternative. An advantage for US studies is that using serum cotinine facilitates direct comparison with representative data for US non-smokers from the National Health and Nutrition Examination Survey (NHANES).32 ,33
Saliva cotinine and metabolites
Cotinine molecules are small and relatively water soluble with minimal protein binding in the blood, with concentration in saliva that parallels serum, but approximately 15% to 40% higher.34 Thus, the analytical sensitivity in this matrix is approximately equal to serum, and most methods can process either serum or saliva. The primary advantage of salivary cotinine measurements is that is a relatively non-invasive matrix when collecting a blood sample is not feasible, or when requiring multiple measurements over a limited period. Collection procedures are relatively simple and well tolerated. Contamination, though unusual, is clearly a greater risk for saliva samples than for serum samples. Issues of age, gender, race, oral pH, type of diet, dehydration, or drug treatment and their effect on salivary cotinine concentrations should be addressed for variability.
Hair nicotine and metabolites
Hair nicotine has been used and validated as a biomarker of SHSe among children and adults.35–38 Nicotine is incorporated into hair if it is present in the circulation, and environmental contamination of nicotine is minimal after washing samples.35 ,37 The hair growth rate is 1 cm/month, 1 cm of hair proximal to the scalp represents the last month's exposure.39 Hair can characterise exposure and time; theoretically, 10-cm of hair represents 10 months of past exposure, a longer period than covered by other biomarkers. The average hair nicotine dose (cumulative exposure/duration of exposure) is therefore less affected by daily variability from fluctuating exposure, varying metabolism and elimination of nicotine.40
The sensitivity of hair nicotine methods varies (LODs range 0.02–0.2 ng nicotine/mg hair).19 ,41 ,42 LC-ultraviolet (UV),42 or GC-MS41 ,43 can be used for hair nicotine determination. Hair nicotine concentrations are highly reproducible over 1 year, proving the method's sensitivity for detecting individual changes in smoking habits and SHSe.43
Advantages of the hair matrix include collection ease, storage at room temperature without degradation for up to 5 years, and shipping without special handling.35 ,44 Scarcity of hair can preclude using this approach for infants or some adults. Chemical hair treatments, however, can reduce hair nicotine concentration by 9% to 30% and relevant information should be collected with hair samples for any necessary adjustments.45 ,46 Similar to most biomarkers, gender and race may play roles in determining hair nicotine concentrations. Hair colour could likely influence nicotine concentrations, since nicotine is bound to melanin and the type and amount of melanin in hair varies with hair colour. However, Zahlsen et al 47 found that nicotine uptake did not differ due to hair colour or thickness, or person's age or gender. Among children, younger children have higher hair nicotine concentrations than older children, with the same SHSe.45 ,48
Toenail nicotine and metabolites
Toenail nicotine concentration shows promise for SHSe assessment, as it reflects relatively long exposure periods: depending on the length of the clipping, the concentration can represent up to several months of past exposure (toenails grow at a rate of approximately 1 mm/month).49 In one study, toenail nicotine concentrations strongly predicted self-reported exposure, even after 20 years' storage at room temperature.50 Similar to hair samples, toenails can be collected easily and shipped without temperature restrictions.
Toenails are less directly exposed than hair to environmental nicotine, with concentrations only reflecting nicotine taken up from blood circulation by nails during growth. Slow toenail growth rates overcome day-to-day exposure variability and provide potentially more stable estimates of average exposure, which is critical to assessing long-term exposures.50 ,51 In one study, toenail nicotine concentrations were significantly correlated with reported tobacco smoking (r=0.63).52 They are also predictive of high and low SHSe among non-smokers50; in newborns, fingernails and toenails have been used to determine nicotine exposure in utero.53
Population data on toenail biomarker concentrations are not available, and determining normative population concentrations is critical to relating these concentrations to disease risk and tobacco exposure. Given the low nicotine concentration per mg of toenail, collecting clippings from all 10 toenails is recommended. Age is inversely related to toenail nicotine concentrations in women, even after controlling for cigarette consumption and SHSe frequency,54 while nail fungus infection and nail polish do not appear to influence concentrations or exposure. No data exist on toenail nicotine concentrations relating to gender or race. Laboratory methods to analyse nicotine in toenail samples include high-performance liquid chromatography-electrochemical detection (HPLC-ECD) (LOD of 0.1 ng/mg toenail),19 or GC-MS (LOD range 0.025–0.01 ng/mg toenail).55 ,56
NNK metabolites in urine
NNK is a tobacco-specific lung carcinogen shown to induce adenocarcinoma of the lung in rats, mice, or hamsters.9 Its metabolites, tNNAL can be measured in the urine of SHS-exposed non-smokers. NNK itself is not found in human urine because of its extensive metabolism to NNAL and other metabolites.9 ,57 As NNAL represents only 15% of NNK dose intake, it is prone to interindividual and intraindividual variability due to metabolic variability of the other 85% of metabolites. tNNAL has been quantified in smokers' blood,58 but its measurement in SHS-exposed non-smokers' blood has not been reported and may be too low for current detection methods. Highly sensitive, validated analytical methods available to quantify tNNAL in urine are GC-TEA, GC-MS/MS and LC-MS/MS. LC-MS/MS is currently the assay of choice for NNAL as concentrations can be determined in first morning urine, spot urine and 24 h urine samples.
The major advantages of the tNNAL biomarker are its specificity to tobacco smoke and its direct relationship to a lung carcinogen. NNK is found only in tobacco products—never in the general environment, unless SHS is present. Thus, NNAL detection in urine signifies exposure to, and uptake of, the lung carcinogen NNK. The tNNAL biomarker is well established and it is not detected in the urine of non-exposed individuals. Multiple studies have demonstrated uptake of NNK by SHS-exposed non-smokers, as well as transplacental exposure from smoking mothers by analysing amniotic fluid or first morning urine.59 ,60 NNAL has a longer half-life than cotinine, thus representing longer exposure. However, NNAL's t1/2 of up to 3 weeks is much less than nicotine's half-life in hair or toenail matrices.
The disadvantages of tNNK are the needs for expertise in analytical chemistry and costly equipment. NNAL is carcinogenic and mutagenic and must be handled with extreme caution in the laboratory. To date, the highest tNNAL concentration in the urine have been observed in infants and children with SHSe (80–90 fmol/ml urine), compared to concentrations of 20–50 fmol/ml urine in non-smoking adults.10 ,24 It is not clear whether these are differences in metabolism between children and adults, or in exposure or other factors. One study comparing tNNAL concentrations in teen versus adult smokers did not find significant differences, suggesting that metabolic differences are unimportant, but further study is needed.26 Studies of smokers do not indicate gender differences in tNNAL concentrations; this is also true for exposed non-smokers. There may be racial differences in tNNAL concentrations in smokers' urine, as some studies suggest higher concentrations in African Americans compared to Caucasians.61 ,62 Whether these observations extend to non-smokers is unknown.
Utility of NNK metabolites versus nicotine metabolites
A moderately strong correlation is evident between NNK metabolites and nicotine metabolites in the urine of SHS-exposed non-smokers, with similar results in smokers. In a study of 74 children, as tNNAL increased from 0.05 to 0.35 pmol/ml urine, total cotinine (the sum of cotinine and its glucuronide) increased from 15 to 50 ng/ml urine,25 leaving little doubt that cotinine at these concentrations in non-smokers' urine implies the presence of tNNAL. Since it is easier and less expensive to measure nicotine than tNNAL, one could argue that the latter is not necessary. tNNAL in urine, however, may have greater public health impact and better predictive utility for the adverse health effects of SHSe, compared to detection of nicotine metabolites. This specificity reflects the pulmonary carcinogen NNAL and its parent NNK, although nicotine may be related to carcinogenesis, atherosclerosis, platelet adhesion and coronary heart disease (CHD) vasoconstriction, and has addictive properties and high-dose toxicity. Detection of an actual carcinogen, tNNAL, in non-smokers' urine, signals a hazard, and using this biomarker to discourage smoking (eg, feedback for parents of exposed children) has been proposed. Its detection in the urine of SHS-exposed non-smokers repeatedly attracts media attention, leading to further support for tobacco-free legislation and tobacco control.
Factors affecting concentrations of biomarkers
Age
Differences in drug elimination between children and adults are well documented, particularly during neonatal and early infant periods, when hepatic, renal, cardiac and lung functions are immature.63 ,64 Nicotine and cotinine pharmacokinetics in neonates exposed in utero to nicotine had significantly longer nicotine t1/2 in the infants’ serum than that reported for adults (neonates t1/2 nicotine=11.2 h (95% CI 8.0 to 18.9 h); adults t1/2 nicotine=approximately 2 h) with no differences for cotinine,29 suggesting that differences in the nicotine t1/2 but not in the cotinine t1/2 between neonates and adults may relate to differences in nicotine metabolism.65 Several studies report cotinine elimination in children's urine, and all show younger children having slower elimination and/or higher concentrations than older children or adults66–68; also observed with hair (nicotine and cotinine).45 ,48 ,69
Disease state
Disease states may affect metabolite concentrations in children and adults. Children with asthma show higher cotinine concentrations in hair and urine compared to children without asthma.70 ,71 However, it is difficult to determine if the differences are due to metabolism or exposure as the studies only used parental report to measure environmental exposure.
Other sources of exposure to nicotine
Other sources of nicotine exposure in non-smokers include breastfed infants with smoking mothers, even if the infant does not have SHSe. Children breast fed by mothers who smoke outside the house can also have higher urine cotinine concentrations than bottle-fed children with direct SHSe.72 One study reported Alaska Native children, aged 4 years and older, chewing tobacco.73 Finally, through hand-to-mouth activity, children may ingest floor dust containing nicotine or touch fabrics that have been exposed to SHS, such as smokers' clothing.74
Interpreting biomarker concentrations
Results of quantitative biomarkers' concentrations of SHSe need careful interpretation, which could consider incorporating cut-off points to separate smokers from SHS-exposed non-smokers; using threshold concentrations for significant consequences of SHSe; and assessing the severity of SHSe in non-smokers.
Separating smokers and non-smokers
As cotinine is specific for nicotine exposure, any detectable concentration indicates exposure to tobacco, tobacco smoke, or medicinal nicotine. NNK is also specific for tobacco and is not present in medications or food; any NNAL concentration indicates exposure to tobacco or tobacco smoke.
Daily smokers typically have plasma or serum cotinine concentrations of 100 ng/ml or higher, or urine tNNAL concentrations of 1000 fmol/ml or higher,57 while light or non-daily smokers can have cotinine concentrations below 10 ng/ml. Heavy SHSe may result in plasma cotinine concentrations up to 25 ng/ml.75 Thus, overlap may occur between cotinine concentrations of non-smokers who experience heavy SHSe and light/occasional smokers. The optimal cut-off point should minimise false classification of SHS-exposed non-smokers versus smokers, and will depend on the extent of non-smokers' SHSe and the smoking behaviour of the population's smokers.
The most widely used serum cotinine cut-off point (14 ng/ml) to distinguish smokers from non-smokers is based on work from the early 1980s in England.76 More recently, a cut-off point of 12 ng/ml was determined using UK data (1996–2004) from a representative sample,77 which suggests that SHSe in England did not declined dramatically over 20 years. SHSe in the US is much lower today, however, compared to 1980s England. An optimal cut-off point of 3 ng/ml was determined using NHANES data (1999–2004).78 Due to differences in smoking behaviours and perhaps in cotinine metabolism, the optimal US cut-off point, for adults, varies by race/ethnicity (non-Hispanic whites: 5 ng/ml, non-Hispanic blacks: 6 ng/ml, Mexican–Americans: 1 ng/ml).78 The low cut-off in Mexican–Americans reflects lower SHSe and higher prevalence of light/occasional smoking.
Generalisability from national sampling to particular subpopulations requires careful consideration, particularly if the target population's exposure profile is unusual. Thus, researchers should consider the target population when selecting the optimal cut-off point to separate smokers from non-smokers (eg, casino workers with high SHSe (optimal cut-off point: >3 ng/ml). Hair cotinine cut-off point values in women, pregnant women and children (0.8, 0.2 and 0.2 ng/mg, respectively) have been determined using data from the USA, Canada and France.79 Lack of representative population data for other biomarkers in the different matrices makes prediction of optimal cut-off values difficult for these markers.
Assessing risk from SHSe
For researchers interpreting a particular nicotine, cotinine, or NNAL concentration as a biomarker of risk from exposure, an appropriate comparison group is required. Three possible approaches are available: (1) to consider that any concentration resulting from a specific biomarker of SHSe such as nicotine, cotinine, or NNAL, indicates an increased risk28; (2) to classify exposure with respect to tertile or quartile of a general population of exposed individuals, if available (eg, US NHANES (serum),80 England's Health Survey (saliva),77); and (3) to classify exposure severity based on known environmental exposure (eg, those heavily exposed in bars or casinos) or on association with disease (see Relationship of Biomarkers to Disease Risk).
Interpreting total NNAL concentrations in urine
Average tNNAL concentrations in the urine of SHS-exposed non-smokers range from 18 to 90 fmol/ml urine, while for smokers this figure is 1000 fmol/ml urine or higher.3 ,57 Exceptions occur, however, and there may be some overlap in smokers and non-smokers' NNAL concentrations. No cut point differentiating smokers from non-smokers has been determined.
Based on a 50 fmol/ml urine concentration in SHS-exposed non-smokers, tNNAL excretion is estimated at 75 pmol/day.81 Since tNNAL represents 15% of the NNK dose, NNK exposure in SHS-exposed non-smokers is estimated at 500 pmol/day, or a dose of about 1.1 mg (0.01 mg/kg) in 30 years of SHSe. The lowest total dose of NNK shown to induce lung tumours in rats is 1.8 mg/kg,9 or 200 times higher than the dose of a SHS-exposed non-smoker.
Another method is to compare tNNAL concentrations in SHS-exposed non-smokers to tNNAL concentrations in smokers. In one study, tNNAL concentrations in the urine of SHS-exposed women were 5.6% of that in the urine of their partners who smoked.82 Epidemiological studies estimate that the excess risk for lung cancer in SHS-exposed women is about 20% higher than that for unexposed women83, or 1% to 2% of the excess risk for lung cancer in smokers (1400% to 1900%) compared with non-smokers, a figure consistent with the 5.6% relative NNAL concentrations.82
Relationship of biomarkers to disease risk
Two criteria for valid biomarkers of risk are that they predict disease risk and that a change in biomarker concentration corresponds to a change in disease risk. Such research is problematic because most SHSe-related diseases take years to develop, and established biomarkers, such as cotinine, measure short-term exposure. If, however, measuring a biomarker at a particular time reflects chronic SHSe over a longer period, then a quantitative relationship between biomarker concentrations and disease may exist.
SHSe is a known cause of CHD.28 Several studies among adults have found a positive relationship with cotinine levels and CHD prevalence (170%; p<0.05),84 and CHD development (50%; p<0.05).85 Toenail nicotine concentrations were associated with CHD events among SHS-exposed nurses,54 although this association was not statistically significant, possible because of drastic SHSe reduction in US hospitals between 1982 and 1998.
SHSe causes respiratory disease among children.28 Higher saliva cotinine concentrations were associated with doubling the ‘tendency for colds to go to the chest’ and reduction of lung function markers in children.86 Among adults with asthma or chronic obstructive pulmonary disease (COPD), higher urine cotinine was associated with greater COPD severity and lower physical health status and disease-specific quality of life.87 COPD outcomes, however, were not associated with self-reported SHSe or personal badge nicotine concentrations. Urine NNAL concentrations is a better predictor of SHSe effects among COPD subjects.88 The risk of asthma-related hospital admissions in adults increased with higher hair concentrations of nicotine, but not cotinine.89 Significant decreases in lung function and increases in inflammatory markers were observed after acute SHSe (1 h), particularly in men.90 In addition, although the effects on lung function appear to disappear within 60 min; inflammatory cytokines remain elevated for at least 3 h after SHSe.
SHSe reduces birth weight28 and cotinine levels of SHS-exposed pregnant women have been statistically significantly associated with reductions in mean birth weight (range: 73 g to 200 g).28 ,91–93 Non-significant associations have found with hair nicotine levels.94 ,95
Conclusions
This article summarises the current scientific evidence on the use of biomarkers to measure SHSe, analytical methods, biological matrices and their interpretation. Cotinine is the biomarker of choice for measuring SHSe in urine, blood and saliva. Nicotine can be measured in hair and toenails. NNK is a tobacco-specific lung carcinogen. NNAL, a NNK metabolite, represents only 15% of the NNK dose intake but can be detected in the urine of SHS-exposed non-smokers. Use of each biomarker has its advantages and disadvantages, making selection dependent on the study's objectives, subjects, design and setting, funding, issues of privacy, invasiveness and subject's age. The length of SHSe may result in selecting hair or toenails over biofluids. The information provided here may assist investigators in selecting the optimal biomarker when designing their study.
Acknowledgments
The authors would like to thank Nicole Ammerman and Charlotte Gerczak for their technical and editing assistance, respectively. The authors would also like to thank Drs Benjamin Apelberg, Dana Best, Michael Cummings, Geoffrey Fong, Lara Gundel, Katherine Hammond, Melbourne Hovell, Andrew Hyland, Jonathan Klein, Neil Klepeis, Robert McMillen, James Repace, and Jonathan Winickoff for their participation in the expert meeting.
References
Footnotes
-
Funding This work was supported by the Flight Attendant Medical Research Institute's grant for the Centers of Excellence at Johns Hopkins; the University of California, San Francisco Bland Lane; and the American Academy of Pediatrics Julius B Richmond. The funding organisation had no role in the preparation of the manuscripts.
-
Competing interests None declared.
-
Provenance and peer review Not commissioned; externally peer reviewed.