Article Text
Abstract
Objectives To measure the short-term effects of an electronic nicotine delivery device (“e cigarette”, ENDD) on desire to smoke, withdrawal symptoms, acceptability, pharmacokinetic properties and adverse effects.
Design Single blind randomised repeated measures cross-over trial of the Ruyan V8 ENDD.
Setting University research centre in Auckland, New Zealand.
Participants 40 adult dependent smokers of 10 or more cigarettes per day.
Interventions Participants were randomised to use ENDDs containing 16 mg nicotine or 0 mg capsules, Nicorette nicotine inhalator or their usual cigarette on each of four study days 3 days apart, with overnight smoking abstinence before use of each product.
Main outcome measures The primary outcome was change in desire to smoke, measured as “area under the curve” on an 11-point visual analogue scale before and at intervals over 1 h of use. Secondary outcomes included withdrawal symptoms, acceptability and adverse events. In nine participants, serum nicotine levels were also measured.
Results Over 60 min, participants using 16 mg ENDD recorded 0.82 units less desire to smoke than the placebo ENDD (p=0.006). No difference in desire to smoke was found between 16 mg ENDD and inhalator. ENDDs were more pleasant to use than inhalator (p=0.016) and produced less irritation of mouth and throat (p<0.001). On average, the ENDD increased serum nicotine to a peak of 1.3 mg/ml in 19.6 min, the inhalator to 2.1 ng/ml in 32 min and cigarettes to 13.4 ng/ml in 14.3 min.
Conclusions The 16 mg Ruyan V8 ENDD alleviated desire to smoke after overnight abstinence, was well tolerated and had a pharmacokinetic profile more like the Nicorette inhalator than a tobacco cigarette. Evaluation of the ENDD for longer-term safety, potential for long-term use and efficacy as a cessation aid is needed.
Trial registration No.12607000587404, Australia and New Zealand Clinical Trials Register
- E-cigarette
- electronic nicotine delivery device
- withdrawal
- pharmacokinetics
- cessation
- nicotine products
Statistics from Altmetric.com
Nicotine replacement therapies (NRTs) are safe and effective aids for smoking cessation1; however, they remain underutilised.2 To widen their appeal to smokers, a variety of effective NRTs and cigarette smoking alternatives are needed. In 2004, a Beijing-based company, Ruyan Group (Holdings) Ltd, China, patented and launched an electronic nicotine delivery device (ENDD) or “e-cigarette”,3 a battery-powered device resembling a cigarette that contains a microelectrical circuit activated by drawing on the mouthpiece. With each puff, a small amount of nicotine-propylene glycol solution contained in replaceable cartridges is heated and vapourised, to create a visible mist without smoke or flame. Manufacturers now export a growing number and variety of these devices to industrialised countries. People report buying them to help quit smoking, to reduce cigarette consumption and costs, to relieve tobacco withdrawal symptoms due to workplace smoking restrictions, or as a replacement for cigarette smoking.4
Although ENDD use has not been comprehensively studied, to our knowledge, no deaths or hospitalisations from ENDD use have been reported. Analyses of emissions of the Ruyan V8 ENDD suggest a low risk of toxicity (M Laugesen, 2009, TC2009/034355, submitted). The risk of impurities in the cartridge liquid is, however, of concern to the US Food and Drug Administration,5 and there is a lack of internationally certified manufacturing sites. Regulators also lack data on the ability of ENDDs to relieve the desire to smoke cigarettes and to suppress other tobacco withdrawal symptoms. Study designs that assess change in subjective withdrawal symptoms have been used to evaluate the utility of other novel nicotine delivery devices.6–8 Using such an approach, this study compared the Ruyan V8 ENDD containing 16 mg nicotine cartridges to one containing 0 mg cartridges (identical in appearance and in chemistry but lacking nicotine), Nicorette nicotine inhalator and usual cigarettes. We measured change in desire to smoke, withdrawal symptoms, product preferences, serum nicotine levels and adverse events after 1 day's use.
Methods
Participants
Participants were recruited from the local community in Auckland, New Zealand, between January 2008 and February 2008. They were assessed via an initial telephone call for eligibility (aged between 18 and 70 years, smoked 10 or more factory-made cigarettes per day for at least the past year, smoked their first cigarette of the day within 30 min of waking, and were not currently attempting to quit smoking or wishing to do so in the next 30 days). Eligible participants were invited to visit the study centre at The University of Auckland to give written informed consent, complete a questionnaire asking about demographic and smoking characteristics and undergo screening (medical history, blood pressure, heart rate and urinalysis for glucose). We excluded people who reported recent myocardial infarction, angina pectoris or other serious medical conditions (diabetes mellitus, severe allergies, poorly controlled asthma or other airways disease, poorly controlled psychiatric disorders or current chemical dependence other than nicotine) and pregnancy (confirmed by positive urinary dipstick for βHCG), breastfeeding, blood pressure >180 mm Hg systolic and/or 100 mm Hg diastolic, weight <45 or >120 kg, or current use of any other smoking cessation medications. At this time, we invited all participants to indicate if they were also willing to give venous blood samples for analysis of nicotine.
Study medication
Ruyan V8 ENDDs supplied by the manufacturer, Ruyan Group (Holdings) Ltd, are pen-shaped devices that include a rechargeable lithium ion battery, an electronic circuit, a vaporiser, a replaceable cartridge and a mouthpiece. The cartridge labelled 16 mg contains 1.06 g of liquid comprising 8.8% water, 1.4% nicotine, 89.7% propylene glycol and 0.1% glycol (M Laugesen, 2009, TC2009/034355, submitted). Participants randomised to a day using the ENDD were asked to puff the device as they would their usual cigarette for 5 min. After the first hour, they left the study centre and used the device as required for a further 8 h before returning to complete the rating scales. We charged all batteries 2 weeks before the study and between each day of use to ensure they would operate as intended. Nicorette inhalators (manufactured at the time of the study by Pfizer Health AB, Helsingborg, Sweden) were purchased commercially. When participants used the inhalator, we gave them a blister pack of six inhalator cartridges, each containing 10 mg of nicotine for inhalation, with instructions to puff on the inhalator over 20 min in the first hour. Thereafter, they were instructed to use it freely, preferably hourly, up to a maximum of six cartridges over the day, as recommended by the manufacturer,9 before returning to the study centre. When randomised to smoke their usual cigarettes, participants did so over the first 5 min in the first hour, then freely as they wished.
Sample size
Based on a previous study,10 we estimated 48 participants would be needed to detect a one-point difference in desire to smoke on an 11-point (0–10) scale measured at 20 min, for the comparison between the 16 and 0 mg ENDD, assuming a within-participant SD of the response variable of 1.5 points and statistical power of 90% at a two-sided significance level of 5%.
Procedures
Participants were requested to abstain from smoking and alcohol from 20:00 on the night before each study day and from food and caffeine for at least 1 h before the session. On arrival at the study centre, carbon monoxide (CO) was measured in participants' expired breath using a Bedfont Smokelyser.11 If CO was ≤15 parts per million (ppm), the assigned study treatment was allocated; however, if CO was >15 ppm or they reported smoking in the previous 12 h, participants were rescheduled wherever possible to a subsequent session. On the first study day, participants were randomised to use one of four different products: ENDDs containing nicotine (16 mg) or placebo (0 mg) capsules, Nicorette nicotine inhalator or their usual cigarette. Allocation was performed using a random sequence of four codes, each corresponding to one product, prepared in advance by the study statistician using the Latin-square method to control for time effects. Participants and investigators were blinded only to assignment to the ENDD condition (16 or 0 mg), and it was not possible to change the order of treatment allocation. Participants sat at desks in a room where they completed ratings of desire to smoke and other withdrawal symptoms 15 and 5 min before using their allocated product (these ratings were later averaged to provide baseline ratings). They rinsed their mouth with water (to equalise oral pH) and took their first dose at 08:30. When allocated to smoke their usual cigarettes, participants did so outside (required by law); however, when allocated to the ENDDs, participants used them indoors. Ratings were made at 5, 10, 15, 20, 25, 30, 40, 50 and 60 min counting from the first puff on each product. Participants then left the study centre with instructions to continue their usual daily activities but not to smoke (unless in the usual cigarette group) and to use the study product regularly and freely throughout the day. They returned at 17:30 for CO measurement, to report product use including adverse events and the number of any cigarettes smoked, rate the degree of satisfaction and usefulness, and to return the remaining study product. Participants were allowed to smoke as they wished once these measures were collected and during the 3-day washout period between each study day. On the day allocated for using their own cigarette, participants did not take part in evening measurements.
A subset of participants gave venous blood samples for analysis of plasma nicotine concentrations. In this group, in addition to the procedures described above, on each study day, medical staff inserted an 18-gauge butterfly needle in a non-dominant forearm vein and withdrew 5-ml blood samples at baseline, then at 5, 10, 15, 30 and 60 min after initial dosing. Blood samples were held in vacuum tubes in a temperature-monitored cool box maintained between 0°C and 6°C and at the end of each study session were transported to a local laboratory for separation. The plasma fraction was frozen before being shipped to a national reference laboratory for nicotine concentration assay using the method developed by Mahoney and Al-Delaimy.12
Following study completion, participants were invited to attend a group cessation clinic run over 5 weeks. The majority attended at least one of these sessions.
Measures
We assessed withdrawal using three items from the Minnesota Nicotine Withdrawal Scale13: irritability, restlessness and difficulty concentrating. The other Minnesota Nicotine Withdrawal Scale items—increased appetite/weight gain, depression or insomnia—were not included because these would not be plausibly experienced in such a short period. We included an additional item, “desire to smoke” (a cigarette), a construct equivalent to the more commonly used terms “craving” or “urges”,14 which was measured by asking, “Right now, how much do you want a cigarette?” We asked participants to indicate their perception of all items by circling a visual analogue scale number between 0 and 10, where 0=“not at all” and 10=“extremely”. Participants noted the occurrence of adverse events commonly experienced with NRT use using categories adapted from Hajek et al15 and any other reactions not on this list. On the last study day, still blind to the ENDD condition, participants were asked to rate their satisfaction with the products compared to their usual cigarettes using scales (0=“completely unsatisfying”, 10=“fully satisfying”)15; on the same scale, they rated helpfulness (in keeping them from smoking), how pleasant the product was to use, how embarrassing it was to use in the company of others, the degree to which they would use it to aid a quit attempt, and whether they would recommend it to a friend who wanted to stop smoking.
Statistical analysis
All analyses were undertaken on an intention-to-treat basis. We analysed the primary outcome, comparison of change in desire to smoke between the 16 and 0 mg ENDD, using area under the curve over 60 min as the dependent variable. The treatment effect was thus the average change in desire to smoke over the 60 min after first using each product. For this and the other ratings, the baseline score (the area under the curve at 5 min before product use), treatment effect and time period were fixed effects in the model. We also examined the change in desire to smoke between the ENDD and other products (inhalator and usual cigarette) using analysis of covariance with within-participant variation included as a random effect. We dealt with missing data using the last value carried forward method. Pharmacokinetic parameters (Cmax (peak plasma concentration) and tmax (time to reach Cmax)) were calculated based on plasma concentration-time data by model-independent methods using SAS version 9.2 (SAS Institute). Multiple comparisons between products were adjusted for using the Tukey-Kramer method and p values and 95% confidence intervals (CIs) were estimated for each comparison.
Results
Participant characteristics
Figure 1 shows participant flow. Forty participants were randomised, 53% of whom were women. The mean age was 47.6 (SD 12.4) years and the mean level of nicotine dependence (Fagerström Test of Nicotine Dependence16) was 5.4. Participants smoked an average of 20.2 (SD 7.3) cigarettes smoked per day. Twenty-five participants had previously used nicotine patches, nineteen reported previously using nicotine gum, two had used the inhalator, but none had used an ENDD before. During the study days, most participants used the products regularly and abstained from smoking as instructed; however, when using the 16 mg ENDD, participants smoked on average 2.8 usual cigarettes over the day, compared with 4.5 cigarettes when using the 0 mg ENDD and 3.4 cigarettes when using the inhalator. Nine participants on average gave blood samples for pharmacokinetic analysis on each of 5 study days.
Participant flow.
Desire to smoke and withdrawal symptoms
Figure 2 shows the change from baseline in desire to smoke over 60 min. Usual cigarette use resulted in significantly greater reductions in desire to smoke compared to ENDD (16 and 0 mg) and inhalator. Desire to smoke ratings reached their lowest level 5 min after the first puff with usual cigarettes, 10 min after the first puff with 0 mg ENDD, 15 min after first puff with 16 mg ENDD and on completing 20 min of puffing on the inhalator. Over the 60 min period, participants using the 16 mg ENDD experienced a greater decrease in desire to smoke (2.6 units) than participants using the 0 mg ENDD (1.8 units), a difference of 0.82 units (95% CI 0.25 to 1.38; p=0.006) (see table 1). In the comparisons between 16 and 0 mg ENDD at each time point, the difference in desire to smoke became significant from 25 min through to 60 min, when users recorded 1.3 units (95% CI 0.33 to 2.25, p=0.009) less desire to smoke with the 16 mg ENDD than the 0 mg ENDD.
Change in desire to smoke from baseline over the first hour after each product use.
Primary comparisons of change in desire to smoke and other withdrawal symptoms from baseline between 0 and 16 mg nicotine ENDD
Over 60 min, the use of the 16 mg ENDD reduced ratings for irritability, restlessness and difficulty concentrating more than the 0 mg ENDD; however, these differences were not statistically significant (table 1). Similarly, there was no difference in withdrawal symptoms between the 16 mg ENDD and inhalator, whereas usual cigarettes reduced withdrawal ratings more on all items than the other products.
Results of the secondary analyses comparing the 16 mg ENDD, inhalator, usual cigarette and 0 mg ENDD are shown in table 2. Although a greater reduction in desire to smoke was observed with the 16 mg compared to the 0 mg cigarette, this difference was no longer significant when adjusted for multiple comparisons.
Secondary analyses using multivariate* comparisons of change in desire to smoke from baseline between all products
Product preferences
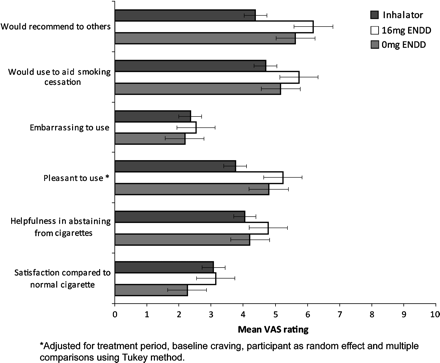
The 16 mg ENDD rated higher for pleasantness of use than the inhalator, by 1.49 units (95% CI 0.23 to 2.74, p=0.016). Figure 3 shows that mean satisfaction ratings for 16 mg ENDD and inhalator were similar and were higher than those for placebo ENDD, and embarrassment associated with using the device in the company of others was low but not significantly different to the inhalator. The 16 mg ENDD was favoured over other products for enabling participants to keep from smoking, to be used as a potential quitting aid and for recommending to a friend who wanted to stop smoking. It was also the most preferred alternative to cigarettes: 58% of participants said they preferred the ENDD, 25% preferred the inhalator and 13% liked neither. The degree of ease of use was similar for ENDD and inhalator (48% and 45%, respectively).
A higher rating is indicative of a greater likelihood of finding the product more satisfying, helpful, pleasant, embarrassing, likely to be used for aiding smoking cessation and to be recommended to others (error bars represent standard error).
Pharmacokinetics
On average, the use of the 16 mg ENDD resulted in modest increases in blood nicotine levels (see table 3). The fastest time to peak nicotine concentration was obtained with usual cigarettes, followed by nicotine ENDDs and the inhalator. Usual cigarette use gave a significantly higher Cmax than the other products in the individual product and crossover analysis, with adjustment for multiple comparisons (p=0.01).
Pharmacokinetic properties of usual cigarette, 16 mg ENDD and Nicorette inhalator
Adverse events
The most frequently reported adverse events were mouth and throat irritation (table 4), and were most common with the inhalator (88%) and least common with the 0 mg ENDD (22%). The differences between active and placebo ENDDs and inhalator were statistically significant (p<0.001). Nausea was most commonly reported after 16 mg ENDD use, but, as with the other between-product differences in adverse events occurrence, was not significant. No serious adverse events (ie, deaths or events requiring hospitalisation) occurred during the study.
Adverse events reported by participants after 9 h of product use, by product
Discussion
The 16 mg Ruyan ENDD was significantly more effective than the 0 mg ENDD at reducing desire to smoke over 1 h of use. The 0 and 16 mg ENDD relieved desire to smoke within the first 10 min of use, but the 16 mg ENDD reduced desire to smoke most at 15 min (10 min after the last puff), more than either the 0 mg ENDD or the nicotine inhalator, which was still being puffed as per instructions given to participants. The reduction in desire to smoke in the first 10 min of ENDD use appears to be independent of nicotine absorption, and may be due to behaviours and oral and tactile sensations similar to cigarette smoking, and the anticipation of nicotine delivery.
In the pharmacokinetic analyses, the serum nicotine Cmax for usual cigarettes was comparable with other studies.17 The 16 mg ENDD's performance was consistent with findings from intensive-mode smoking machine tests of this same make of ENDD, which delivered 10% of the nicotine per puff delivered by a regular Marlboro cigarette (M Laugesen, 2009, TC2009/034355, submitted). This suggests that it is more like a NRT product, concerning nicotine delivery, than a cigarette. The shorter tmax of the ENDD in comparison to the inhalator may reflect some absorption via the respiratory tract compared with buccal absorption for the Nicorette inhalator. The ENDDs in the study were not as consistent for puffing and nicotine delivery as the medicinal Nicorette inhalator. About one-third of participants showed no increase in blood nicotine when using the ENDD. Some participants reported that the device sometimes failed to produce mist when puffed. It is possible that technical problems could have affected the dose received. This is not altogether surprising given that these devices are not manufactured to the same standards required of pharmaceutical devices, such as the inhalator.
We also noted that three participants (one using the inhalator, two using ENDDs) had high baseline serum nicotine values (12.8, 12.0 and 6.6 ng/ml), which declined over the hour, raising the possibility of recent smoking of a tobacco cigarette, despite CO readings suggesting otherwise. Excluding these data in a post hoc analysis on the primary outcome and withdrawal symptoms gave a small increment in the mean decrease in desire to smoke, from 0.82 to 0.91 units (95% CI 0.29 to 1.53), which was still significant (p=0.006) compared to placebo, with little change to the values and significance of changes in irritability, restlessness and poor concentration.
Overall, the Ruyan ENDD was similar to the Nicorette inhalator on a range of subjective ratings of user preferences, and users reported a similar frequency of most adverse events except for a far lower occurrence of mouth or throat irritation than for the inhalator.
The strengths of this study include the study population of dependent but “healthy” male and female smokers recruited from the community; the use of a crossover design to minimise variability, bias and confounding; and the use of items from validated instruments for measuring key outcomes. We used a range of statistical tests including mixed model analysis, which has the advantage of including all the data and accounting for within-subject correlation.
The study has a number of limitations. First, it was statistically powered to detect a difference between 16 mg ENDD and 0 mg ENDD, not to detect a difference between 16 mg ENDD and inhalator, and the desired sample size of 48 was not achieved despite best endeavours to recruit to this target. Second, low baseline ratings of desire to smoke may have limited the degree of observable change. Third, the study was limited to smokers not intending to quit, which may underestimate the reduction in desire to smoke expected in smokers intending to quit. Fourth, this was the first experience for participants and investigators with the ENDD and there was no period of familiarisation with the products before use nor did we test them to ensure normal functioning. Both factors could have affected the pharmacokinetic results and attenuated the ratings of desire to smoke. Fifth, the four-fold greater puffing time allowed for the inhalator is problematic for comparing products, as it is by no means clear yet whether an ENDD inhaling session should be for 5, 10 or 20 min a session. Finally, use of each study product for just 9 h did not allow sufficient time for assessment of delayed acute or chronic use effects, nor of the potential for long-term use.
Conclusions
The Ruyan V8 16 mg ENDD reduced desire to smoke more than the placebo ENDD and during 9 h of use was well tolerated, acceptable to most users, rated significantly more pleasant to use than the inhalator, and in the first hour exhibited a pharmacokinetic profile more like the inhalator than a tobacco cigarette, without excess adverse events. These findings suggest potential to help people stop smoking in the same way as a nicotine inhalator. Our findings should be regarded as preliminary and need to be confirmed for this and other brands of ENDD. The nicotine pharmacokinetics of ENDDs should be confirmed in other studies, and different ways of using these devices need to be explored. A large, well-conducted randomised trial is needed to evaluate the ENDD's efficacy as a quitting aid and to identify any delayed or long-term adverse effects.
What this study adds
The Ruyan 16 mg nicotine ENDD reduces desire to smoke.
The ENDD has a pharmacokinetic profile more like the nicotine inhalator than a tobacco cigarette.
Further research is needed on the pharmacokinetics, long-term use potential, safety, manufacturing quality and quitting efficacy associated with ENDD use.
Acknowledgments
We thank the people who took part, and Joanne Lorimer, Denise Miller and Henry Bohte for their help in undertaking this study.
References
Footnotes
Funding This project was funded by Ruyan Group (Holdings) Limited, Beijing and Hong Kong, via Health New Zealand Ltd. The study sponsors supplied the ENDDs used in the trial and funded the trial. The Clinical Trials Research Unit contracted with Health New Zealand Ltd to conduct the trial, independently of Ruyan Group (Holdings) Ltd. The trial design conduct, analysis and interpretation of results were conducted independently of the sponsors.
Competing interests CB, ST and RL have no competing interests to declare. HM has received honoraria for speaking at research symposia and received benefits in kind and travel support from, and has provided consultancy to the manufacturers of smoking cessation medications. ML acted as contract manager with the sponsor, Ruyan Group (Holdings) Limited, via his company Health New Zealand Ltd. ML and Health New Zealand Ltd have no patent, stock or other financial interest in Ruyan Group (Holdings) Limited or any nicotine or tobacco company. MG has provided consultancy to the manufacturers of smoking cessation medications.
Ethics approval Ethical approval for the trial was obtained from the New Zealand Ministry of Health's Northern Y Ethics Committee (approval number NTY/07/10/109).
Provenance and peer review Not commissioned; externally peer reviewed.