Article Text
Statistics from Altmetric.com
Background
Heated tobacco products (HTP) have an electrical heating component, like e-cigarettes, that heats processed tobacco to 350°C releasing volatile components that often are not detectable in e-cigarettes.1 Although many combustion by-products may be eliminated in HTP devices, nitrosamines are generated in the process of tobacco curing rather than during combustion, and may be transferred from the HTP into the aerosol that it generates.2–4 We hypothesised that HTP may be a significant source of tobacco-specific nitrosamines (TSNA). This pilot study determined TSNA yields in aerosol emitted from HTP in comparison to the electronic and tobacco cigarettes.
Methods
HTP (IQOS; Amber, tobacco flavour), e-cigarettes (MarkTen; 3.5% nicotine, tobacco flavoured) and tobacco cigarettes (Marlboro Red 100) were tested using a Borgwaldt LX-1 smoking machine following the Health Canada Intense protocol (55 mL puff volume, 2 s duration, 30 s interval). Using this puffing protocol, we generated aerosol from a single HTP HeatStick (12 puffs), single tobacco cigarette (8 puffs) and from e-cigarette (55 puffs). We used different number of puffs for each product to achieve a comparable nicotine delivery across all tested products. Cambridge filters (44 mm) were used to capture the total particulate matter from all tested products. The control samples (blanks) were generated by passing 55 puffs of air through the filter. Cambridge filters were spiked with deuterated internal standards and extracted using 20 mL 100 mM ammonium acetate. The following TSNAs were measured using liquid chromatography-tandem mass spectrometry: N'-nitrosoanabasine, N'-nitrosoanatabine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N'-nitrosonornicotine (Toronto Research Chemicals; Canada).5 A limit of quantitation for each compound was 0.5 ng/filter. Nicotine was measured using gas chromatography with nitrogen-phosphorous detector (GC-NPD) method as described previously.6 Each product was tested in triplicate. The average TSNA yields for each product were calculated per single puff and per puffing session. We used analysis of variance to test for statistical differences between the three tested products and t-tests to compare TSNA yields from HTP with yields detected in e-cigarettes and combustible cigarettes.
Results
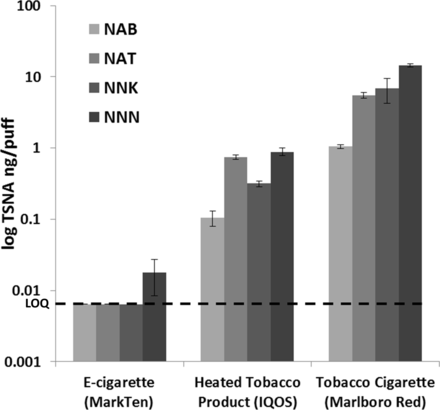
All four TSNA compounds analysed were detected in the HTP. The yields of individual TSNA per puff in the HTP aerosols were 8–22 times lower than in tobacco cigarette smoke (figure 1; all p<0.05). HTP delivered 1.4±0.2 mg nicotine from a single HeatStick (12 puffs); e-cigarette 1.3±0.2 mg per 55 puffs; and a single combustible cigarette 2.1±0.1 mg (8 puffs). TSNA yields normalised per nicotine delivery were also significantly higher in the HTP than those found in e-cigarettes and significantly lower than those found in tobacco cigarettes, except for NNK (p<0.05). TSNA yields in a single tobacco cigarette were between 7 and 17 times higher than TSNA yields in a single HTP HeatStick. No TSNAs were detected in the air control samples.
Yields of tobacco-specific nitrosamines (TSNA) (per puff) in aerosols generated from IQOS heated tobacco product (12 puffs/HeatStick), MarkTen e-cigarette (55 puffs) and smoke from Marlboro Red 100 combustible cigarettes (8 puffs/cigarette). The data presented are log transformed. LOQ, limit of quantitation; NAB, N'-nitrosoanabasine; NAT, N'-nitrosoanatabine; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NNN, N'-nitrosonornicotine.
Conclusions
Like combustible products, HTPs emit substantial levels of carcinogenic TSNA. Although HTP emits lower amounts of TSNA than combustible cigarettes, the amounts are significantly higher than from e-cigarettes. Our findings are consistent with prior reports.3 4 7 One limitation of this study is that one puffing protocol was used for all devices. While this was helpful in comparing TSNA and nicotine delivery between devices, machine-based measurements are not represented of human smoking patterns or constitute intake.8 9 The tested HTP does not reduce emissions of an important class of tobacco carcinogens to the same degree as other commercially available technologies.
Footnotes
Contributors MLG contributed to the conception of the work. MLG and NJL contributed to data analysis. AMM, MNP, MLG, NJL and RJO drafted the manuscript. AMM, MNP and NJL ran all experiments. All authors approved the final version of the manuscript. MLG has full access to all study data and takes responsibility for the integrity of the data and accuracy of the data analysis.
Funding Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers P01 CA200512 and P30 CA016056.
Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests MLG reports grants from and served as an advisory board member to pharmaceutical companies that manufacture smoking cessation drugs. RJO was a member of the FDA Tobacco Products Scientific Advisory Committee, which considered Philip Morris International’s modified risk application for IQOS in January 2018. Other authors declare no conflict of interest.
Patient consent Not required.
Provenance and peer review Not commissioned; externally peer reviewed.