Article Text
Abstract
Assessing tobacco use intensity allows researchers to examine tobacco use in greater detail than assessing ever or current use only. Tobacco use intensity measures have been developed that are specific to tobacco products, such as asking smokers to report number of cigarettes smoked per day. However, consensus on electronic cigarette use intensity measures that can be used for survey research has yet to be established due to electronic cigarette product and user behavior heterogeneity. While some survey measures that attempt to assess electronic cigarette use intensity exist, such as examining number of ‘times’ using an electronic cigarette per day, number of puffs taken from an electronic cigarette per day, volume of electronic cigarette liquid consumed per day, or nicotine concentration of electronic cigarette liquid, most measures have limitations. Challenges in electronic cigarette measurement often stem from variations across electronic cigarette device and liquid characteristics as well as the difficulty that many electronic cigarette users have regarding answering questions about their electronic cigarette device, liquid, or behavior. The inability for researchers to measure electronic cigarette use intensity accurately has important implications such as failing to detect unintended consequences of regulatory policies. Development of electronic cigarette use intensity measures, though not without its challenges, can improve understanding of electronic cigarette use behaviors and associated health outcomes and inform development of regulatory policies.
- electronic nicotine delivery devices
- non-cigarette tobacco products
- addiction
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Self-report surveys are the approach used most commonly to examine tobacco-related knowledge, attitudes, beliefs and behaviours. One advantage of using surveys in tobacco research is that if consistent survey items and response options are used across studies and years, researchers can compare the results of one study with other studies to identify changes over time in tobacco use. For example, in 1964 the first US Surgeon General’s Report on the Health Consequences of Smoking1 was published. At that time, more than 50% of men and 30% of women were current smokers, defined as those who had smoked at least 100 cigarettes in their lifetime and reported ‘currently’ smoking.2 In 2018, 15.6% of men and 12.0% of women in the USA were current smokers (ie, defined as having smoked at least 100 cigarettes in their lifetime and currently smoked cigarettes ‘every day’ or ‘somedays’). Importantly, because nearly identical measures of current cigarette use were used across the 44-year span, researchers are able to document the immense progress that has been made in reducing cigarette smoking prevalence in the USA. Similar core survey items for other tobacco products, such as cigars, waterpipe, smokeless tobacco and electronic cigarettes (e-cigarettes), have been developed and used to examine ever use and current use of tobacco products. Data from surveys that use these measures can be used to monitor trends over time, identify priority areas for research and inform regulatory policy. However, there are not yet standard e-cigarette use measures due, in part, to surveys needing to adapt to the evolving marketplace of e-cigarette products.
Tobacco use intensity measures
While useful for examining prevalence, measuring ever and current tobacco use has limitations. For example, both an individual who smokes 20 cigarettes every day and an individual who smokes 1 cigarette per week can be considered ‘current’ smokers, despite greatly differing behaviours and exposures to toxicants in cigarette smoke. Thus, survey items have also been developed and used to measure tobacco use intensity (eg, cigarettes smoked per day). These measures serve many purposes, such as identifying more dependent tobacco users or identifying differing levels of risk associated with tobacco use. For example, while other factors are also important, research shows that those who smoke on more days and smoke more cigarettes per day have higher levels of dependence3 and greater risk for negative health outcomes4 5 than those who smoke fewer cigarettes or smoke on fewer days. Comparing cigarette smokers based on cigarette smoking intensity measures relies on the key assumption that all cigarettes are approximately the same. That is, each cigarette contains comparable amounts of tobacco leaf with similar nicotine content, thus exposing users to comparable amounts of the dependence-causing chemical nicotine and other toxicants. This assumption is reasonable for cigarettes because while some changes in cigarette design and smoking behaviour have occurred over time6 and cigarettes are not uniform, the nicotine content, cigarette size and cigarettes per pack are similar across brands.7 Although differences in these elements between different types of tobacco products (eg, cigars, cigarettes, smokeless tobacco) present challenges in comparing use across users of different tobacco products, tobacco use intensity survey measures allow for comparisons between users of the same tobacco product.
Common methods and challenges to assessing e-cigarette use intensity
As e-cigarette use has increased in recent years,2 8–17 ever and current e-cigarette use have been examined in surveys using items similar to those used for cigarettes. While the use of numerous terms to describe e-cigarettes (eg, electronic cigarettes, e-cigarettes, electronic nicotine delivery systems, vapes and so on) presents challenges for ensuring that researchers and participants are referring to the same products when developing and answering survey questions, the use of pictures and preambles that describe all products considered to be e-cigarettes can improve assessment of e-cigarette use.18 Some have called for consensus measures to be established for e-cigarette use18 and work has been conducted by researchers participating in the National Institutes of Health and the Food and Drug Administration’s (FDA) Tobacco Centers of Regulatory Science grant programme to identify core items that might be used to assess key e-cigarette use domains. Importantly, e-cigarette measurement must account for the great heterogeneity of e-cigarette devices on the market, including disposable ‘cigalikes’ that resemble cigarettes, refillable ‘vape pens’, variable wattage ‘box mods’, ‘pod mods’ that use disposable cartridge/pods filled with e-cigarette liquid, and disposable vapes that resemble computer flash drives, to name a few of the many device type categories. While core items for measuring e-cigarette current and ever use have been identified,19 the group noted that the development of survey measures to assess e-cigarette use intensity presents a more challenging problem, precisely due to e-cigarette device and liquid heterogeneity. The purpose of this commentary is to discuss possible approaches to measuring e-cigarette use intensity and challenges associated with each approach, as well as to offer considerations for researchers who aim to examine e-cigarette use intensity in the future.
Puff topography
One approach to measuring e-cigarette use intensity is to examine the number of puffs taken from an e-cigarette per day. There are approximately 10–15 puffs in a single combustible cigarette20 and standard procedures have been developed to examine toxicant emissions associated with puffs taken from a single cigarette.21 As a result, researchers can calculate approximate daily puff counts for cigarette smokers based on the number of cigarettes smoked per day. However, this approach cannot be used for e-cigarettes. Unlike cigarette smokers who typically smoke a cigarette from start to finish in a single session, e-cigarette users often puff from the same e-cigarette in multiple sessions, with sessions not being consistent in total puff duration or number of puffs.20 While some researchers attempt to address this issue by asking e-cigarette users to report number of puffs per day, this approach presents challenges. Research is needed to verify whether participants can recall accurately puffs taken per day. Some puff-activated device product marketing claims that a single cartridge contains an approximate number of puffs (such as 20022), though products may not provide a consistent number of puffs across devices due to poor manufacturing standards or counterfeit devices.23 Additionally, some e-cigarette devices that use a button to activate the heater include ‘puff counters’ that record each time the button has been pressed, but these data also needed to be studied to determine their accuracy. Indeed, self-reported times per day and device puff counters appear to be correlated moderately, but some e-cigarette users appear to provide extreme/not reliable self-reported values24 and not all devices use puff counters.
However, even if the validity of these approaches is confirmed, relying on number of puffs per e-cigarette cartridge, puff counters or participant recall of puffs per day are problematic because the length of each session, number of puffs per session and individual puff characteristics vary considerably. While puff duration can vary for other tobacco products, like cigarettes, ultimately total puff duration of a cigarette is limited by the amount of tobacco that can be burned in the cigarette. Therefore, cigarettes smoked per day remains a viable option for examining cigarette smoking intensity. E-cigarettes allow for greater variation in puffs, both in duration and number, which complicates comparing puffs between users. Indeed, laboratory studies where detailed topography data can be collected demonstrate this: one study found that average e-cigarette puff volume ranges from 96.81 to 133.92 mL,25 whereas another study reported average puff volumes ranging from 331.2 to 519.6 mL.26 Although these studies used similar protocols (10-puff directed bouts) and only varied devices and liquid characteristics, some puffs were more than five times larger than others .
Number of e-cigarette use sessions per day
Another approach to assess e-cigarette use intensity is to ask participants to report the number of ‘times’ they use their e-cigarette or the number of use sessions per day. This approach allows researchers to compare e-cigarette users by number of sessions per day regardless of device type. Indeed, some surveys have attempted to do this and even define a session/time (eg, ‘assume that one ‘time’ consists of around 15 puffs or lasts around 10 minutes’27) and report on e-cigarette use times per day as a measure of e-cigarette use intensity or frequency (eg, Refs. 24 and 28) with greater intensity associated with greater dependence. However, e-cigarette use and cigarette smoking have important differences. Because a cigarette must be lit, cigarette smokers must either smoke an entire cigarette in a single session or extinguish and relight the same cigarette. Previous research suggests less than half to as many as 69% of smokers report ever relighting their cigarettes,29 30 though this behaviour may not occur regularly among those who do relight.30 However, because e-cigarettes are activated by puffing or by using an on/off switch and button, e-cigarettes are used intermittently on a regular basis. Thus, e-cigarettes more readily allow for use sessions that can range from a single puff to hundreds of puffs.
E-cigarette users may also engage in different use patterns depending on the situation. For example, some surveys indicate that e-cigarette users may ‘vape more before going into a situation where vaping is not allowed’31 such as right before entering a building. Other studies have noted that many e-cigarette users’ behaviours are ‘far from homogenous’ and users have ‘difficulty tracking their own use’.32 Qualitative data demonstrate this heterogeneity of behaviours with some experienced e-cigarette users reporting using e-cigarettes more or less frequently compared with cigarette smoking, some inhaling more deeply and others less deeply compared with cigarette smoking, and some reporting they take longer puffs compared with cigarette smoking.33 Because an e-cigarette use session could be dependent on the user, device type and characteristics, situation or other factors, defining a standard e-cigarette use session between, or even within, e-cigarette users is challenging.
Amount of e-cigarette liquid consumed
Almost all e-cigarette liquids contain the same primary ingredients (propylene glycol, vegetable glycerin, nicotine and chemical flavorants34), although in different concentrations. Some researchers have used survey items assessing amount of liquid used per day as a measure of e-cigarette use intensity. Using this approach, it might be assumed that higher amount of liquid used per day is associated with greater use intensity and thus exposure to nicotine or other toxicants. However, even if the liquids have the same concentration of nicotine and other compounds, this approach cannot account for the effects of highly variable e-cigarette device characteristics across the range of e-cigarettes available, which can have dramatically different abilities to aerosolize liquid. In a study comparing differences between e-cigarette users based on device type, ‘third generation’ (eg, box mod) e-cigarette users reported using 2.5 times more e-cigarette liquid per week than ‘second generation’ (eg, vape pen) e-cigarette users. However, third generation device users’ cotinine levels were only 1.4 times higher than second generation device users, likely due to the fact that third generation device users’ average e-cigarette liquid nicotine concentration was 4.1 mg/mL vs 22.3 mg/mL for second generation device users.35 These data demonstrate that amount of liquid consumed may be a useful indicator of amount of aerosol inhaled by users, but not necessarily an accurate measure of exposure to nicotine and other toxicants in the aerosol. Thus, when considering e-cigarette intensity measures, researchers must determine whether their goal is to assess quantity of use or exposure to aerosol emitted from e-cigarettes. Additionally, validation is needed to determine whether e-cigarette users can accurately quantify the amount of e-cigarette liquid they consume in a given amount of time.
Nicotine concentration in e-cigarette liquid
Because nicotine is the primary dependence causing substance in e-cigarettes, some researchers have focused on examining exposure to nicotine as a measure of e-cigarette use intensity. One approach is to measure nicotine in e-cigarette liquids in addition to volume of liquid consumed. Survey items have been developed to examine the content of e-cigarette liquid, specifically the concentration of nicotine. For example, the Population Assessment of Tobacco and Health Study asks e-cigarette users if the e-cigarette ‘you use contain[s] nicotine’ and ‘what concentration of nicotine do/did you use?’.36 This question presents challenges as the labelling of nicotine content varies across e-cigarette products and liquids (eg, only a number provided without context, concentrations in milligrams of nicotine per millilitre of solution (mg/mL) or a percentage of the total volume of the liquid) and may be difficult to interpret if units are not provided. For example, ‘3’ is a feasible nicotine concentration in mg/mL or per cent nicotine, but 3 mg/mL and 3% represent nicotine concentrations that differ by a factor of 10. Another concern is that e-cigarette liquid nicotine concentrations may be labelled incorrectly.37 Some liquids advertised as nicotine free have been found to contain quantifiable amounts of nicotine and nicotine labelling may differ by 5%–20% of actual nicotine concentrations in liquids,38 further complicating attempts to assess nicotine exposure. Additionally, users may mix homemade e-cigarette liquids (ie, ‘do-it-yourself’ liquids) resulting in unknown nicotine concentrations or inconsistent concentrations between batches.
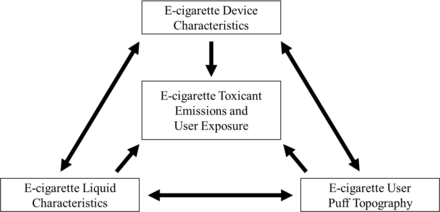
In cases where the assumption can be made that e-cigarette users know the nicotine concentration in their liquid, assessing e-cigarette liquid nicotine concentration may be useful for assessing nicotine exposure. Previous reports indicate that most experienced or regular e-cigarette users report they know their e-cigarette liquid nicotine concentration,39 even though they may not be fully aware of how nicotine concentration relates to nicotine ‘strength’.40 Laboratory research demonstrates that when holding other factors constant, increased nicotine concentration in e-cigarette liquid results in increased nicotine exposure for users.41–43 While some e-cigarette users associate lower nicotine concentration in e-cigarette liquid with lower nicotine exposure,44 in real world settings, a higher liquid nicotine concentration is not necessarily associated with greater nicotine exposure or greater e-cigarette use intensity. A study found that users of higher power e-cigarette devices used liquids with mean nicotine concentrations that were 5.4 times lower than lower power devices, but higher power e-cigarette device users had cotinine levels (a metabolite of nicotine) that were 1.4 times higher than users of lower power devices.35 This is because nicotine yield from an e-cigarette (and therefore user nicotine exposure45) is dependent on the e-cigarette device, liquid characteristics and user behaviour,46 rather than e-cigarette liquid nicotine concentration alone (see figure 1).
Schematic demonstrating how e-cigarette (1) device characteristics, (2) liquid characteristics and (3) user puff topography interact and influence e-cigarette toxicant emissions and user exposure directly. Bidirectional arrows show how changes in one factor are often associated with changes in other factors. A given combination of e-cigarette device and liquid characteristics and user puff topography is associated with specific toxicant emissions and user exposure.
Implications for accurate e-cigarette intensity measurement
These challenges in developing accurate e-cigarette intensity measures have many implications. For example, the inability to measure e-cigarette intensity may impact clinical laboratory researchers’ ability to ‘screen potential participants, report participant use history, and study factors that influence user toxicant exposure’.47 E-cigarette use intensity measurement challenges also complicate the development and evaluation of regulatory policies. For example, the European Union established a policy that prohibited the sale of e-cigarette liquids with nicotine concentrations greater than 20 mg/mL with the goal of limiting e-cigarette users’ nicotine exposure by only allowing ‘delivery of nicotine that is comparable to the permitted dose of the nicotine derived from a standard cigarette…’.48 To examine the impact of this policy, surveys that measure e-cigarette use intensity solely by examining e-cigarette liquid nicotine concentration in products used before and after the policy may not yield an accurate picture of changes in nicotine exposure, due to e-cigarette device heterogeneity. That is, if e-cigarette users who used devices containing liquids with nicotine concentrations greater than 20 mg/mL transitioned to devices that contained liquids with nicotine concentrations of less than 20 mg/mL, the policy may be viewed as effective in decreasing nicotine exposure. However, data demonstrate that nicotine delivery from devices that operate at higher electrical power (in watts) using liquids with nicotine concentrations of 4 mg/mL can result in nicotine delivery that exceeds that of a cigarette.35 In this scenario, without an understanding of the relationship between nicotine delivery and device characteristics, e-cigarette users, researchers and policy makers may perceive erroneously that reducing e-cigarette liquid nicotine concentration will result necessarily in a decrease in nicotine exposure. Furthermore, high powered devices that can expose users to large amounts nicotine from low nicotine concentration liquids also expose users to greater concentrations of toxicants relative to lower power devices49 and may result in unintended adverse health effects.50 Thus, a nicotine concentration limiting policy may cause e-cigarette users to inhale more aerosol and hence increase toxicant exposure. Indeed, compensatory puffing behaviours that are associated with increased carcinogenic carbonyls and increased exposure to formaldehyde51 were recorded among e-cigarette users who were assigned to use an e-cigarette device with adjustable wattage using a liquid with nicotine concentration lower than their usual liquid.52 In order to more fully understand the implications of a policy limiting nicotine concentration in e-cigarette liquids, studies should assess changes in e-cigarette use intensity before and after the policy was implemented.
Another issue is that researchers and public health professionals looking to help e-cigarette users decrease their e-cigarette use may have difficulty in determining whether e-cigarette users are reducing their consumption, especially if users transition between e-cigarette products. Additionally, if common measures cannot be used across devices researchers may have difficulty in identifying e-cigarette devices that put users at greatest risk for dependence. For example, how does one compare the e-cigarette use intensity and dependence between a user of a pod-based 4.08 W e-cigarette device who uses one pod per day that contains 0.7 mL of liquid with a nicotine concentration of over 69 mg/mL53 54 with an e-cigarette user of a ‘box mod’ device that operates at 71.6 W with a liquid nicotine concentration of 4 mg/mL and uses 7.8 mL of liquid per day (as in Ref. 35)? Current survey measures used to assess e-cigarette use intensity are likely insufficient and further research is needed to inform the best approaches for comparing e-cigarette use intensity across device types.
Summary and future directions for e-cigarette use intensity measurement
There is urgent need to measure e-cigarette use intensity. Due to the extreme device heterogeneity that is a hallmark of the e-cigarette product class, developing a set of standard items that assess e-cigarette use intensity equally well across all products may be challenging. Survey items that measure e-cigarette user puff topography, number of use sessions, amount of e-cigarette liquid consumed or e-cigarette liquid nicotine concentration all have limitations. Future studies that use these measures with others in combination may be most effective for assessing e-cigarette use intensity.
As of December 2020, FDA is reviewing e-cigarette product applications submitted to the agency as part of the Premarket Tobacco Product Application process. FDA marketing authorisation decisions may lead to some consolidation of the number and variety of e-cigarettes available on the market, but a wide range of products is likely to remain available. This will continue to present challenges for measuring e-cigarette use intensity. In order to understand e-cigarette use intensity, three factors must be considered simultaneously: (1) device characteristics, (2) liquid characteristics and (3) user behaviours. As illustrated in figure 1, e-cigarette emissions and user exposure are all influenced by these three factors. Importantly, with e-cigarettes, all three of these factors can be modified. Using nicotine emissions as an example, a given combination of e-cigarette device settings (eg, device wattage), liquid ingredients (eg, nicotine concentration) and user behaviours (eg, puff duration) is associated with specific and predictable55 nicotine emissions. Importantly, there are numerous combinations of e-cigarette device, liquid and user behaviour characteristics that can be employed to achieve a given quantity of nicotine emitted from an e-cigarette. However, unlike combustible cigarettes, e-cigarettes allow users to modify virtually all the factors that impact emissions. Thus, regulations that focus on only one or two of these factors will have difficulty in achieving desired outcomes, especially when many e-cigarette devices are ‘open-systems’56 .
Researchers may need to use a combination of survey measures that better capture the interaction between e-cigarette devices, liquid characteristics and use patterns given their influence on e-cigarette use intensity and toxicant exposure. Additionally, the specific survey items to capture these measurement domains will vary according to study purpose and population. Novel approaches and methods may also improve the ability to obtain data necessary for examining e-cigarette use intensity when used in combination with self-reported survey methods. One approach may be to incorporate the use of user-uploaded images or videos to surveys, for example, asking e-cigarette users to provide images of their devices and liquids. When feasible, researchers may consider asking participants to provide videos or demonstrations of ‘typical’ puffs if puff counters are to be used which would allow researchers to extrapolate total puff duration in a given period of time. Combining device and liquid characteristics obtained from images and typical puffing behaviours obtained from videos might enable more accurate calculation of the amount of aerosol, nicotine and toxicant exposure for individual e-cigarette users.
In laboratory settings, researchers may also examine if any of the currently available survey measures are more associated with biomarkers, such as plasma nicotine, urine cotinine, urine propylene glycol or vegetable glycerin, or other toxicants found in e-cigarette aerosol. These data may inform which survey measures are most useful for comparing e-cigarette use intensity and exposure to e-cigarette aerosol and toxicants. Finally, e-cigarette use intensity comparisons between users or timepoints may be most useful when as many factors are held constant as possible, such as using the same measures over time and only comparing users of a single e-cigarette device and liquid combination.
What this paper adds
Surveys are used to measure tobacco use intensity by researchers for many purposes.
Measures of tobacco use intensity have been developed for tobacco products, such as number of cigarettes smoked per day.
Despite attempts to develop electronic cigarette use intensity measures, great heterogeneity in electronic cigarette device and liquid characteristics and user behaviour make measuring electronic cigarette use intensity challenging.
This paper describes the challenges, limitations and implications of the current methods used to measure electronic cigarette use intensity.
Researchers should consider electronic cigarette device and liquid characteristics as well as user behaviours when attempting to measure electronic cigarette use intensity.
Ethics statements
Patient consent for publication
References
Footnotes
Contributors ES wrote the first draft of the manuscript. All authors provided feedback on the first draft and approved the final version of the manuscript.
Funding Funding for this work was supported by the Food and Drug Administration (FDA), Centre for Tobacco Products (CTP) and the National Institutes of Health (NIH). ES was supported by National Institute on Drug Abuse (NIDA) grant number U54DA036105. MB-T and SM were supported by National Cancer Institute (NCI) grant number U54CA228110. SP was supported by NIDA grant U54DA046060, and JBU was supported by NCI grant number U54CA180905. KW and RG were not supported by grant funding for their contributions to this manuscript. All authors contributed substantially to the writing of this manuscript.
Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH or the FDA.
Competing interests ES is named on a patent application for a smartphone app that determines electronic cigarette device and liquid characteristics.
Provenance and peer review Not commissioned; externally peer reviewed.