Article Text
Abstract
Objective To examine the population effectiveness of nicotine replacement therapies (NRTs), either with or without professional counselling, and provide evidence needed to better inform healthcare coverage decisions.
Methods A prospective cohort study was conducted in three waves on a probability sample of 787 Massachusetts adult smokers who had recently quit smoking. The baseline response rate was 46%; follow-up was completed with 56% of the designated cohort at wave 2 and 68% at wave 3. The relationship between relapse to smoking at follow-up interviews and assistance used, including NRT with or without professional help, was examined.
Results About one-fourth of recent quitters at each wave reported to have relapsed by the subsequent interview. Odds of relapse were unaffected by use of NRT for >6 weeks either with (p=0.117) or without (p=0.159) professional counselling and were highest among prior heavily dependent persons who reported NRT use for any length of time without professional counselling (OR 2.68).
Conclusions This study finds that persons who have quit smoking relapsed at equivalent rates, whether or not they used NRT to help them in their quit attempts. Cessation medication policy should be made in the larger context of public health, and increasing individual treatment coverage should not be at the expense of population evidence-based programmes and policies.
- Smoking cessation
- nicotine replacement therapy
- cohort studies
- public policy
- smoking-caused disease
- fire safety
- prevalence
- taxation and price
- environmental tobacco smoke
- harm reduction
- advertising and promotion
- advocacy
- anti-tobacco media campaigns
- cessation
Statistics from Altmetric.com
- Smoking cessation
- nicotine replacement therapy
- cohort studies
- public policy
- smoking-caused disease
- fire safety
- prevalence
- taxation and price
- environmental tobacco smoke
- harm reduction
- advertising and promotion
- advocacy
- anti-tobacco media campaigns
- cessation
Introduction
Smoking cessation medications are recommended in the US Department of Health and Human Services clinical practice guidelines1 and have been available over the counter since 1996.2 Yet previous declines in adult smoking prevalence have stalled in the past 5 years,3 and the ‘quit ratio’ index of smoking cessation in the population (ratio of former smokers to ever-smokers) has not changed since 1998,4 while one in five adults continues to smoke.3 The smoking cessation guidelines are based on the demonstrated efficacy of medications with and without behavioural counselling in randomised controlled trials (RCTs), as summarised in a meta-analysis of RCTs, which found estimated ORs (odds ratios) of effectiveness ranging from 1.5 to 3.1.1 ,5
However, the generalisability of the results of clinical trials to the community setting has been challenged, and the RCT findings contrast with cross-sectional population studies, which have observed ORs of 0.316 and 0.79,7 favouring less relapse among persons not using cessation medication. Other representative population studies have also found no beneficial effect of the use of nicotine replacement therapy (NRT) on quitting success8 ,9 or long-term abstinence compared with unaided cessation10–12 and no improvement with their availability in cigarette consumption,13 annual smoking cessation rates13–15 or smoking prevalence.15 Beard et al found increased short-term abstinence only among persons who had reported using NRT six months earlier.15a The US Food and Drug Administration's (FDA) new Regulatory Science initiative calls for ‘scientific methods employing empirical and causal evidence utilised in the evaluation and approval of all the products that the FDA regulates’,16 underscoring the importance of population studies as well as RCTs. In light of these observations, better evidence of population effectiveness of NRTs is needed to inform healthcare coverage decisions for smoking cessation.
The present study assesses the effects of NRT use on smoking abstinence when used alone or in combination with behavioural counselling in a longitudinal cohort of recent quitters. The specific hypotheses examined are (1) NRT used for the recommended duration (ie, >6 weeks), either with or without professional counselling, will lower the likelihood of smoking relapse; (2) NRT used for >6 weeks in combination with professional counselling will be more effective in reducing smoking relapse than use of NRT alone and (3) NRT used for ≤6 weeks will not be effective in reducing relapse.
Methods
Study design and sample
Between January 2001 and June 2002, professional telephone interviewers at the Center for Survey Research, University of Massachusetts Boston, obtained a probability sample of 6739 Massachusetts adults, with an over-sampling of adult smokers, young adults (between ages 18 and 30) and recent quitters (those who quit smoking in the past 2 years). At wave 1, 66% of residential households were successfully screened and 70% of eligible adults were interviewed for an overall response rate of 46%. Between January 2003 and July 2004, attempts were made to re-interview all 4991 of the adults in the wave 1 sample who were current smokers, recent quitters or young adults. Interviews were completed with 2805 respondents, for a follow-up rate of 56%. Between January 2005 and July 2006, attempts were made to re-interview all 2805 respondents to the wave 2 interview. Interviews were completed with 1916 adults (68%). The analytic sample for this report consists of the individuals who at either wave 1 or 2 reported having quit smoking during the prior 2 years and who completed the interview at the subsequent wave.
Data and measures
The primary outcome measure was smoking relapse by the second and third waves of this survey among those who reported quitting in the first and second waves, respectively. The primary predictors were derived from respondents' reports of the type of help, if any, that they used in order to quit smoking. Participants were asked whether they had used nicotine replacement in the form of patch, gum, inhaler or nasal spray to help them quit, and if so, what was the longest period of time that they had used the product continuously. They were also asked whether they had joined a quit smoking programme or received help from a doctor, counsellor or other professional.
Covariates included length of abstinence reported at the beginning of the 2-year period (<6 months or between 6 months and 2 years) and nicotine dependence in the year before quitting with a high level of dependence defined as smoking within 30 min of waking up in the morning and consuming ≥20 cigarettes per day. Nicotine dependence was also controlled in a separate analysis using ordinal variables for both time of first cigarette after waking up and number of cigarettes smoked per day before quitting. Additional covariates were gender and age group (18–44 years or older than 44 years), which were retained in the models as potential confounders; race (Caucasian or non-Caucasian) and education (tested in both ordinal and dichotomous forms), which were retained in models only if found to be statistically significant or confounders of the main predictors.
Statistical analysis
Contingency table analyses were conducted with the Pearson χ2 statistic to test the association between each predictor variable and relapse ascertained at the subsequent survey wave. Multilevel logistic regression analyses were conducted, with the unit of analysis the wave-to-wave (2-year) transition, that is, between the first survey and first follow-up, and between the first and second follow-up. Generalised linear latent and mixed models multilevel logistic modelling was performed to account for intra-individual correlation, while controlling for the potential confounders. All statistical analyses were conducted with weighting to account for probability of selection and to adjust for attrition in respective waves.
Results
Relapse to smoking
A total of 787 surveyed adults reported having quit smoking within the 2 years prior to the wave 1 interview (‘recent quitters’). Of these, 480 (61.0%) completed the second wave interview. One-fourth (25.2%) of these reported to have relapsed to smoking by the second wave interview. A total of 364 adults who completed the second wave interview reported having recently quit smoking. Three-fourths (78.2%) of those who completed the second wave interview also completed the third wave interview (n=285), and 27.4% of these reported having relapsed to smoking by the third wave interview. Contingency table analyses comparing the characteristics of responders to wave 2 were more likely to be Hispanic than responders to wave 1, but not otherwise different with or without professional help, level of dependence, length of abstinence, household smoking policy, smoking status of spouse, gender, age, education level, race, marital status or income. Responders to wave 3 were more likely than responders to wave 2 to be older but were not otherwise different with respect to the remaining features.
Use of smoking cessation therapy
Frequencies and duration of use of NRT to quit at waves 1 and 2 are shown in table 1. About one in five recent quitters at wave 1 (22.6%) and at wave 2 (20.4%) reported having used a nicotine replacement product in their quit attempts. However, only 7.5% of recent quitters (33.2% the NRT users) had used it for >6 weeks at wave 1 and 13.3% of recent quitters (34.8% of NRT users) had used it for >6 weeks at wave 2 (table 1).
Use of NRT among recent quitters
Results of contingency table analyses found that the only significant predictor of relapse among recent quitters from the first to the second wave interview was the duration of abstinence at wave 1: those who reported having been abstinent for <6 months were more than twice as likely to relapse than those who had been abstinent for >6 months (35% vs 17%, p<0.001) (table 2). Relapse among recent quitters from the second to the third wave interview was significantly higher among persons who had used NRT for any length of time without professional help (52%) compared with those who had used NRT for any length of time with professional help (30%) or persons who did not use NRT at all (22%) (p=0.015) (table 2).
Predictors of smoking relapse at waves 2 and 3
Factors associated with relapse to smoking
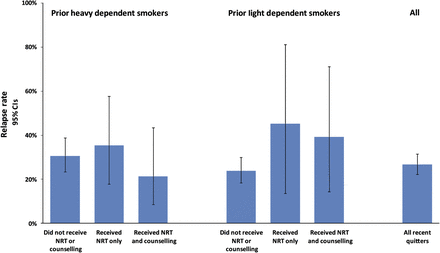
Mean unadjusted relapse rates to smoking and 95% CIs are shown in figure 1 for heavy and light dependent smokers who did not receive NRT or counselling, received NRT only or received NRT and counselling. Results of generalised linear latent and mixed models analysis of all transitions, from waves 1 to 2 and from waves 2 to 3, found that the odds of smoking relapse was unaffected by use of NRT for more than 6 weeks either with (p=0.117) or without (p=0.159) professional counselling or by use of NRT for any length of time either with (p=0.398) or without (p=0.073) professional counselling (table 3). The odds of smoking relapse were lowest among persons who reported abstinence for 6 months or longer (OR 0.46, 95% CI 0.29 to 0.73) and highest among prior heavily dependent persons who reported NRT use for any length of time without professional counselling (OR 2.68, 95% CI 1.40 to 5.11) (table 3). When ordinal variables (time of first cigarette after waking up and number of cigarettes smoked per day before quitting) were substituted in the models for nicotine dependence prior to quitting, the odds of smoking relapse were higher among persons who reported NRT for more than 6 weeks without professional counselling (p=0.014) as well as among persons who reported use of NRT for any length of time without professional counselling (p=0.031).
Mean relapse rates among prior smokers unaided or using nicotine replacement therapy (NRT) used for more than six weeks with or without counselling.
NRT and other factors associated with likelihood of smoking relapse
Results of sensitivity analyses among persons who had quit smoking either within the past year or within the past 6 months also showed no statistically significant differences except for higher relapse rates among prior heavily dependent persons who used NRT for any length of time without professional counselling and also among prior light dependent persons who had quit smoking within the past year and who used NRT for more than 6 weeks without professional counselling (table 3).
Discussion
The results of this representative study of recent quitters raise serious questions regarding the population-level effectiveness of widely popular smoking cessation medications used with or without behavioural counselling. The main finding is that persons who quit smoking relapsed at equivalent rates, whether or not they used NRT to help them in their quit attempts, in clear distinction to the results of RCTs. A major challenge to the applicability of smoking cessation trials to the community setting is their selection criteria which have been found to exclude 66% of participants with nicotine dependence and 59% of a subgroup of nicotine-dependent persons motivated to quit.17 For example, the common restriction of trials to heavier smokers may impair those studies' external validity and result in selection bias.18
The lack of support from population research for evidence from clinical trials has been theorised to result from greater effectiveness of cessation medication among heavier smokers enrolled in clinical trials than among lighter smokers in the population.18 The present study, however, finds that heavily dependent smokers who used NRT without also receiving professional help were twice as like to relapse compared with those who did not use NRT. This may indicate that some heavily dependent smokers perceive NRT as a sort of ‘magic’ pill, and upon realising it is not, they find themselves without support in their quitting efforts, doomed to failure. Such an iatrogenic effect would represent an example of the dangers that Chapman and other observers maintain could result from today's medicalisation and commodification of smoking cessation.7 ,15 ,19–21
The recent trend of treating tobacco dependence in the USA as a clinical condition requiring behavioural counselling and/or medications to help smokers quit is manifest by the rise in sales of NRT products from US$45 million in 1984, when nicotine gum was introduced, to US$129 million in 1991 and then to over US$800 million annually since over-the-counter sales of NRT were approved in 1997.22–24 In addition, US$841 million were realised in sales in 2007 of prescription drugs for smoking cessation.25 Tobacco use prevention at the state level has also increasingly relied upon cessation medication, as the number of state Medicaid programmes covering one or more forms of NRT increased to 39 in 2011 from 17 in 1996.26 ,27
The next important finding of this study is that despite the popularity of NRT, very few quitters are following the recommended practice of using the product for 8 weeks. Although duration of use was not coded in the study in a way that permits estimation of the average length of use, clearly only one-third of users at waves 1 and 2 used it for the recommended period. A similar pattern has been seen in other studies to report duration of use or compliance.11 ,28–34 For example, Pierce et al 11 reported median 14 days and mean 28 days use of NRT among smokers attempting to quit in California.
The main potential threat to validity of the present findings is from loss to follow-up. The follow-up rates of 60.0% at wave 2 and 68.1% at wave 3 are not unusual for a prospective cohort study following subjects for 4 years but do introduce a possibility of a bias due to differential loss to follow-up. Adjustment to the baseline weights using age, gender, race and ethnicity in an iterative raking procedure created weights, which yielded distributions on these demographic variables that were either identical to those at baseline or differed by at most four-tenths of a percentage point. We therefore believe that this threat to validity has been minimised.
While smoking status was not determined on the basis of urinary or blood cotinine, self-report does have an advantage of sensitivity to the full length of time of recall in comparison to biomarkers with relatively short half-lives. Results of sensitivity analyses regarding self-reported NRT use, examined relapse rates among persons who had quit smoking at more recent times prior to survey, suggest that differential recall of use of NRT was unlikely to have biased the results in the direction of no effect among NRT users.
The findings of this study cast doubt on the relative effectiveness of NRT as a population strategy and on the expectations of the effects on smoking prevalence of providing cessation services to individuals. Comprehensive population-based tobacco control programmes led to major achievements in reducing smoking in the USA prior to the focus on cessation medications, including major decreases in consumption in Massachusetts and California on the order of 50%–60%.35 ,36 As total expenditures for cessation medications have increased to over one and a half billion dollars, funding for comprehensive public health programmes has unfortunately plummeted from US$750 million in 2002 to US$457 million in 2012 and is expected to decline further with state budget cuts in 2012.37 Funding ineffective services that aim to change individual behaviour may be resulting in the loss of scarce resources from public health programmes that have proven to be effective in changing social norms and reducing smoking (eg, mass-media counter-marketing campaigns, adoption of comprehensive smoke-free laws, raising the retail price of tobacco products through excise tax increases and restricting advertising and promotion).
In the context of the disappointing trends in smoking prevalence and quit ratios, the present findings provide perspective regarding the role of RCTs as the sole standard for setting guidelines that are applied to population-based interventions. A summary of the key features of, and divergence between, relevant studies reporting results of RCTs and population studies is presented in table 4. While the RCT design provides the best control for confounding and isolating causal mechanisms and is essential for determining clinical effectiveness, the cohorts and clinical settings in which they are conducted may not be generalisable to the population in the ‘real world’.39–42 Early proponents of RCTs as a standard, including Cochrane43 and Bradford Hill,44 acknowledged that the design might be insufficient for determining the effectiveness of an intervention under normal conditions, in a community or in field settings. As the nation embraces an expansion of delivery of healthcare services, care must be taken to establish a credible science base that is based on the combined evidence of RCTs as well as population studies of long-term effectiveness, particularly in the areas of prevention and control of chronic disease.
Randomised controlled trials and population studies of effectiveness of NRT
The US FDA is responsible for the approval of smoking cessation medications. The Food and Drug Administration Amendments Act of 2007 authorises the FDA to require post-marketing surveillance of approved drugs including both observational epidemiologic studies and clinical trials.45 This puts the onus on the FDA and manufacturers of cessation medications to demonstrate the efficacy on individual as well as population levels. Furthermore, under the Family Smoking Prevention and Tobacco Act Section 918, the FDA in consultation with the Institute of Medicine needs to consider relapse prevention in recommending standards for the regulation of ‘fast-tracked’ new cessation medications.46 The results of this study demonstrate that population measures of effectiveness are essential to the agency's carrying out its responsibilities under the law.
Seventy per cent of smokers report wanting to quit, while 45.3% report making a serious quit attempt in the past year.4 ,47 Current healthcare reform legislation in the USA would significantly expand coverage of tobacco cessation under Medicaid, Medicare and private insurance plans in accordance with the Department of Health and Human Services strategy for ending the tobacco epidemic.48 As the nation moves to expand coverage of healthcare services, policies addressing the design, marketing and use of cessation medications should be made in the larger context of public health. Increases in coverage for individual treatment must not be at the expense of public health programmes and policy interventions that have proven effective in promoting cessation.
What this paper adds
Previous declines in adult smoking prevalence have stalled, and the ‘quit ratio’ index of smoking cessation in the population has not changed since 1998, despite the availability of smoking cessation medications. Randomised controlled trial findings concerning nicotine replacement therapy contrast with cross-sectional population studies. In light of these observations, better evidence of population effectiveness of nicotine replacement therapies is needed to inform healthcare coverage decisions for smoking cessation. The present study assesses the effects of nicotine replacement therapy use on smoking abstinence when used alone and/or in combination with a behaviourally counselling in longitudinal cohort of recent quitters.
Acknowledgments
The authors are grateful to Simon Chapman for review and comments regarding a draft of this manuscript and to Paul M Penchalapadu for help with data management and analysis.
References
Footnotes
-
Funding This work was supported by a grant from the National Cancer Institute, State and Community Tobacco Control Interventions Research Grant Program (grant number 2R01 CA86257-05). The sponsor had no role in the study design; collection, analysis and interpretation of data; in the writing of the report or in the decision to submit the paper for publication.
-
Correction notice This article has been corrected since it was published Online First. The Results section of the Abstract now begins: About one-fourth of recent quitters.The first paragraph of Results now reads: Of these, 480 (61.0%) completed the second wave interview. One-fourth (25.2%) of these reported to have relapsed to smoking by the second wave interview. A total of 364 adults who completed the second wave interview reported having recently quit smoking. Three-fourths (78.2%) of those who completed the second wave interview also completed the third wave interview (n = 285), and 27.4% of these reported having relapsed to smoking by the third wave interview. In Table 2, the items under ‘Race’ now read Non-Caucasian and Caucasian, respectively.
-
Competing interests None.
-
Ethics approval University of Massachusetts, Boston.
-
Provenance and peer review Not commissioned; externally peer reviewed.
-
Data sharing statement Data are not available for public sharing.