Article Text
Abstract
Background Waterpipe tobacco package labelling typically indicates “0.0% tar” and “0.05% or 0.5% nicotine”.
Objective To determine the extent to which nicotine labeling is related to nicotine delivery.
Methods 110 waterpipe smokers engaged in a 45-minute waterpipe smoking session. Puff topography and plasma nicotine were measured. Three waterpipe tobacco brands were used: Nakhla (0.5% nicotine), Starbuzz (0.05% nicotine), and Al Fakher (0.05% nicotine). Data were analyzed by one-way ANOVA.
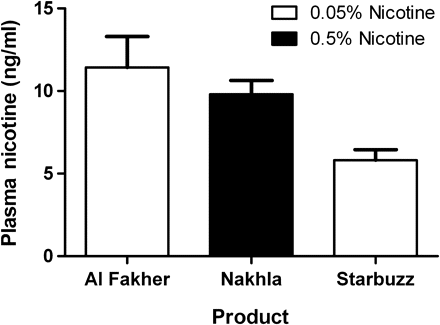
Results Topography did not differ across brands. Peak plasma nicotine varied significantly across brands. Al Fakher had the highest nicotine delivery (11.4 ng/ml) followed by Nakhla (9.8 ng/ml) and Starbuzz (5.8 ng/ml).
Conclusions Nicotine labelling on waterpipe tobacco products does not reflect delivery; smoking a brand with a “0.05% nicotine” label led to greater plasma nicotine levels than smoking a brand with a “0.5% nicotine” label. Waterpipe tobacco products should be labelled in a manner that does not mislead consumers.
- Waterpipe
- nicotine
- tobacco
- narghile
- hookah
- packaging and labelling
- tobacco products
Statistics from Altmetric.com
Introduction
Waterpipe tobacco smoking has become more prevalent globally in the last two decades, in part because many individuals believe that it is not as harmful or addictive as cigarette smoking.1–5 In fact, laboratory evidence suggests that waterpipe tobacco smoke, like cigarette smoke, contains carbon monoxide (CO), nicotine and ‘tar’.6–8 Furthermore, chronic waterpipe use is associated with a decrease in lung function and is genotoxic to lymphocytes.9 10
The perception of less harm from waterpipe tobacco smoking may be perpetuated by waterpipe tobacco product labelling. Ma'assel, a sweetened flavoured tobacco mixture, is a popular form of waterpipe tobacco. Up to 77% of ma'assel packages indicate the percentage of ‘tar’ in the product as 0.0%.11 This information is accurate because, by definition, ‘tar’ is a smoke product and not a tobacco product.12 However, the information is also potentially misleading, as many readers may infer that using the product in a water pipe does not produce ‘tar’ when, in fact, it does.8 In addition, many ma'assel packages are labelled as containing 0.05% or 0.5% nicotine, a label that can be confusing for consumers because no information is provided that details how these percentages were calculated.
Whether the nicotine content labelling on ma'assel packages is relevant to actual user nicotine delivery is unknown. The purpose of this analysis was to examine plasma nicotine concentrations in waterpipe tobacco smokers who used three different brands of waterpipe tobacco during a 45-minute smoking session. We hypothesised that the labelled nicotine content would not predict plasma nicotine concentration consistently.
Method
Participants
A total of 110 participants (32 women, 64 Caucasian) were included in the analysis. Participants ranged in age from 18 to 28 years (mean: 20.8, SD: 2.15), had completed 12–19 years of education (mean: 13.7, SD: 1.47) and reported smoking tobacco in a water pipe between 2 and 30 times per month (mean: 7.0, SD: 7.0) for a duration of 6 months to 5 years (mean: 1.75, SD: 1.15). Of the 110 participants, 89 reported regular use of alcohol, 51 were daily cigarette smokers and 27 reported smoking marijuana in the month prior to participation. Participants were excluded if they reported any recent physical or psychiatric illness, smoking marijuana >5 days per month, drinking alcohol >25 days per month or any other illicit drug use within the last month. All participants gave their written informed consent and studies included in this analysis were approved by the Institutional Review Board of Virginia Commonwealth University (VCU). Sessions took place at the Clinical Behavioral Pharmacology Laboratory on the VCU campus.
Study design and procedures
The data from three studies (one in progress) that used similar methods were combined for the purpose of this analysis.13 14 The ongoing study and the Blank et al study involved two 2-hour sessions in which current waterpipe users smoked active or placebo waterpipe tobacco. In the latter study,14 waterpipe users who also smoked at least five cigarettes per day participated in two 2-hour sessions that involved either cigarette or waterpipe smoking. Only the data from active waterpipe conditions were included in this analysis. For all participants, overnight smoking abstinence was verified at the beginning of each session by an expired air CO level ≤10 ppm. In addition, baseline plasma nicotine levels were below the level of quantitation (ie, 2 ng/ml) for all participants, which is indicative of 12-hour tobacco abstinence. An indwelling venous catheter was then inserted into a forearm vein, physiological monitoring equipment was attached and continuous monitoring of heart rate and blood pressure was commenced. Subjective effects, CO, carboxyhaemoglobin and nitrous oxide were also measured in all three studies. The data are presented elsewhere.13 14
The waterpipe apparatus and smoking procedures were consistent across all studies.14 Briefly, a chrome body was screwed into an acrylic base that was filled with enough water to cover approximately 2.5 cm of the waterpipe body's conduit. A glazed ceramic head was packed with 10 g (n=49) or 15 g (n=61) of product and covered with a circular sheet of aluminium foil that was perforated by a screen pincher (http://www.smoking-hookah.com). A quick-lighting charcoal briquette (Three Kings, Holland) was lit and placed on top of the foil. The leather hose was fitted with puff topography measurement hardware. The hose was fitted with a wooden mouthpiece that was capped by a sterile plastic tip (http://www.hookahcompany.com).
Participants smoked their preferred brand and flavour of waterpipe tobacco. If participants did not have a preferred brand of tobacco, Nakhla was generally used as the default brand. A total of 16 flavours were smoked. Participants smoked water pipes ad libitum for a minimum of 45 min. Puff topography was measured throughout the waterpipe smoking bout, and blood was sampled for later analysis of plasma nicotine concentration (see Breland et al15 for details of plasma nicotine analysis). Blood samples were collected 5, 15, 30 and 45 min13 or 5, 25 and 45 min14 after waterpipe smoking started.
Data analysis
The outcome measures of interest were peak plasma nicotine levels (ie, the maximum nicotine concentration in venous plasma observed following product use) and puff topography (including the number of puffs, total volume and average puff volume). Data were analysed by one-way ANOVA with product as the between-groups factor. Effects were considered significant at p≤0.05. The brands used were Nakhla (n=49), Starbuzz (n=44) and Al Fakher (n=17). In addition, the amount of product packed into the ceramic head was either 10 g (n=49) or 15 g (n=61). To ensure that the amount of product did not affect the outcome of this analysis, a separate one-way ANOVA was conducted with the amount of tobacco (10 or 15 g) as the between-groups factor and peak plasma nicotine level as the dependent variable.
Results
The number of puffs, total volume and average puff volume did not differ significantly as a function of the brand used. Peak plasma nicotine levels varied significantly as a function of the brand used. Peak plasma nicotine levels were highest for the Al Fakher brand (mean: 11.4 ng/ml, SD: 7.7), followed by Nakhla (mean: 9.8 ng/ml, SD: 5.9) and Starbuzz (mean: 5.8 ng/ml, SD: 4.2) (figure 1). Peak plasma nicotine levels also varied by the amount packed in the bowl. The peak plasma nicotine level was generally higher in the 15 g group than in the 10 g group; however, the pattern of results across brands was identical: Al Fakher>Nakhla>Starbuzz.
Peak blood plasma nicotine levels as a function of the brand used and the labelled nicotine content. Y-axis: Peak plasma nicotine level (ng/ml). X-axis (left to right): Al Fakher, labelled as 0.05% nicotine content; Nakhla, labelled as 0.5% nicotine content; and Starbuzz, labelled as 0.05% nicotine content. Error bars represent 1SEM.
Discussion
The results of this analysis demonstrate that ma'assel nicotine content labelling is not related to actual nicotine delivery. While Al Fakher and Starbuzz are labelled as 0.05% nicotine and Nakhla is labelled as 0.5% nicotine (figure 2), the order of nicotine delivery from the highest to the lowest was Al Fakher (mean=11.4 ng/ml), Nakhla (mean=9.8 ng/ml) and Starbuzz (mean=5.8 ng/ml). The observed difference in nicotine delivery across products was not due to differences in puff topography nor was it directly related to the amount of product packed into the head of the water pipe. As noted above, there were no significant differences in the number of puffs, total volume or average puff volume across brands. If the product labelling on these waterpipe tobacco products were related to actual nicotine delivery, a product labelled 0.5% nicotine (such as Nakhla) might be expected to deliver 10 times more nicotine than a product labelled ‘0.05% nicotine’. Obviously, this relationship was not observed in this analysis.
Health warnings and nicotine and tar content labelling on waterpipe tobacco packages purchased from http://www.hookahcompany.com.
Waterpipe tobacco smoke contains ‘tar’, nicotine and CO, as well as carcinogenic polycyclic aromatic hydrocarbons and volatile aldehydes that can cause pulmonary disease.6–8 13 16 17 Labelling that includes wording such as ‘0.05% nicotine’ and ‘0.0% tar’ may lead consumers to believe that waterpipe tobacco smoking exposes them to little or no toxicants. In fact, the WHO has suggested that ‘0.0% tar’ labels on waterpipe products be banned.18 Furthermore, health warning labels on waterpipe tobacco products are either non-existent or barely visible, covering an average of just 3.5% of the total surface area of packages.11 Often, the health warning labels on waterpipe tobacco products refer to cigarettes or smoking in general rather than waterpipe smoking in particular (see figure 2 for health warning and nicotine content labels on Nakhla and Al Fakher packaging).11 Overall, the current state of waterpipe tobacco labelling may confuse more than inform consumers. These products should be regulated and held to the same product testing and labelling standards as other tobacco products (eg, cigarettes) because consumers have the right to accurate and non-misleading information regarding the products that they are using.
The limitations and methodological considerations of this analysis include the retrospective nature, between-groups design and the limited number of products tested. As noted above, three studies were included in this retrospective analysis, none of which were initially designed to examine the differences in nicotine delivery across different products. Therefore, some methodological differences exist across studies, namely participants were not randomised to the brand of waterpipe tobacco and they used different flavours of tobacco. Unforeseen individual differences and group differences may have also contributed to the outcome of this analysis. In addition, although many brands of waterpipe tobacco exist, only three brands were tested. Finally, the brands of waterpipe tobacco tested in the current study were not analysed for nicotine content prior to participant use. This information is also relevant to assess the informational value of product labelling. Nonetheless, the data reported here, in conjunction with previously reported waterpipe smoke toxicant analyses, provide a clear picture of product labelling that is at best misleading.
Acknowledgments
The authors wish to acknowledge the expert technical and medical assistance provided by the staff of VCU's Behavioral Pharmacology Laboratory (Janet Austin, MS, and Barbara Kilgalen, RN) as well as the staff at VCU's Bioanalytical Core Laboratory Service Center. The authors also wish to thank Caroline O Cobb, MS, for comments on a previous version of this manuscript.
References
Footnotes
Funding This research was supported by USPHS grants R01CA103827, R01CA120142 and T32DA007027-34. Other funders: NIH.
Competing interests None.
Ethics approval This study was conducted with the approval of the Virginia Commonwealth University.
Provenance and peer review Not commissioned; externally peer reviewed.